Abstract
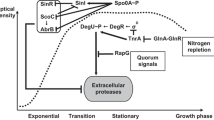
In Clostridium acetobutylicum, abrB310 is transcribed from two transcription start sites (designated A1 and A2) forming an abundant large, and a five- to tenfold less abundant small transcript, respectively throughout exponential, acidogenic growth and early in the transitional period to stationary, solventogenic growth. β-galactosidase reporter vectors were constructed to compare the transcriptional activity of the entire abrB310 promoter and the A1 and A2 transcription start sites individually. In stark contrast to the primer extension data, the A2 start site was threefold more active than the entire promoter, which was threefold more active than the A1 start site in wild type C. acetobutylicum. The activity expressed from all three reporter vectors declined as the cultures transitioned from exponential to stationary growth. In the spo0A-deficient strain SKO1, reporter vector activity continued for 10 h into stationary growth. The removal of the putative Spo0A binding site from all three vectors had no significant effect on promoter activity in either wild type or SKO1. We conclude that the presence of both the A1 and A2 transcription start sites is required for the correct control of abrB310 expression, and that AbrB310 is necessary but not sufficient for the correct transition between acidogenic and solventogenic growth.




Similar content being viewed by others
References
Mitchell WJ (1998) Physiology of carbohydrate to solvent conversion by clostridia. Adv Microb Physiol 39:31–130
Harris LM (2001) Cloning and characterization of the Clostridium acetobutylicum ATCC824 gene encoding the Spo0A transcription regulator and its role in controlling solvent formation and sporulation-specific gene expression. Ph.D. dissertation. Northwestern University, Evanston
Harris LM, Welker NE, Papoutsakis ET (2002) Northern, morphological, and fermentation analysis of spo0A inactivation and overexpression in Clostridium acetobutylicum ATCC 824. J Bacteriol 184:3586–3597
Tomas CA, Alsaker KV, Bonarius HP, Hendriksen WT, Yang H, Beamish JA, Paredes CJ, Papoutsakis ET (2003) DNA array-based transcriptional analysis of asporogenous, nonsolventogenic Clostridium acetobutylicum strains SKO1 and M5. J Bacteriol 185:4539–4547
Alsaker KV, Papoutasakis ET (2005) Transcriptional program of early sporulation and stationary-phase events in Clostridium acetobutylicum. J Bacteriol 187:7103–7118
Sullivan L, Bennett GN (2006) Proteome analysis and comparison of Clostridium acetobutylicum ATCC 824 and Spo0A strain variants. J Ind Microbiol Biotechnol 33:298–308
Scotcher MC, Bennett GN (2005) SpoIIE regulates sporulation but does not directly affect solventogenesis in Clostridium acetobutylicum ATCC 824. J Bacteriol 187:1930–1936
Scotcher MC, Rudolph FB, Bennett GN (2005) Expression of abrB310 and SinR, and effects of decreased abrB310 expression on the transition from acidogenesis to solventogenesis, in Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 71:1987–1995
Strauch MA, Perego M, Burbulys D, Hoch JA (1989) The transition state transcription regulator AbrB of Bacillus subtilis is autoregulated during vegetative growth. Mol Microbiol 3:1203–1209
Strauch MA, Spiegelman GB, Perego M, Johnson WC, Burbulys D, Hoch JA (1989) The transition state transcription regulator abrB of Bacillus subtilis is a DNA binding protein. EMBO J 8:1615–1621
Hahn J, Roggiani M, Dubnau D (1995) The major role of Spo0A in genetic competence is to downregulate abrB, an essential competence gene. J Bacteriol 177:3601–3605
Greene EA, Spiegelman GB (1996) The Spo0A protein of Bacillus subtilis inhibits transcription of the abrB gene without preventing binding of the polymerase to the promoter. J Biol Chem 271:11455–11461
Strauch M, Webb V, Spiegelman G, Hoch JA (1990) The Spo0A protein of Bacillus subtilis is a repressor of the abrB gene. Proc Natl Acad Sci USA 87:1801–1805
Stragier P, Losick R (1996) Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet 30:297–341
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Hartmanis MGN, Gatenbeck S (1984) Intermediary metabolism in Clostridium acetobutylicum: levels of enzymes involved in the formation of acetate and butyrate. Appl Environ Microbiol 47:1277–1283
Zhao Y, Hindorff LA, Chuang A, Monroe-Augustus M, Lyristis M, Harrison ML, Rudolph FB, Bennett GN (2003) Expression of a cloned cyclopropane fatty acid synthase gene reduces solvent formation in Clostridium acetobutylicum ATCC824. Appl Environ Microbiol 69:2831–2841
Mermelstein LD, Welker NE, Bennett GN, Papoutsakis ET (1992) Expression of cloned homologous fermentative genes in Clostridium acetobutylicum ATCC 824. Biotechnology 10:190–195
Green EM, Boynton ZL, Harris LM, Rudolph FB, Papoutsakis ET, Bennett GN (1996) Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142:2079–2086
Burchhardt G, Bahl H (1991) Cloning and analysis of the beta-galactosidase-encoding gene from Clostridium thermosulfurogenes EM1. Gene 106:13–19
Tummala SB, Welker NE, Papoutsakis ET (1999) Development and characterization of a gene expression reporter system for Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 65:3793–3799
Shafikhani SH, Leighton T (2004) AbrB and Spo0E control the proper timing of sporulation in Bacillus subtilis. Curr Microbiol 48:262–269
Acknowledgments
This work was supported by U.S. Department of Agriculture grants 00-35504-9269 and 2006-35504-17294 and National Science Foundation grant BES-0418289.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scotcher, M.C., Bennett, G.N. Activity of abrB310 promoter in wild type and spo0A-deficient strains of Clostridium acetobutylicum . J Ind Microbiol Biotechnol 35, 743–750 (2008). https://doi.org/10.1007/s10295-008-0341-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0341-x