Abstract
The effect of omitting zinc from the influent of mesophilic (30 °C) methanol fed upflow anaerobic sludge bed (UASB) reactors, and latter zinc supplementation to the influent to counteract the deprivation, was investigated by coupling the UASB reactor performance to the microbial ecology of the bioreactor sludge. Limitation of the specific methanogenic activity (SMA) on methanol due to the absence of zinc from the influent developed after 137 days of operation. At that day, the SMA in medium with a complete trace metal solution except Zn was 3.4 g CH4-COD g VSS−1 day−1, compared to 4.2 g CH4-COD g VSS−1 day−1 in a medium with a complete (including zinc) trace metal solution. The methanol removal capacity during these 137 days was 99% and no volatile fatty acids accumulated. Two UASB reactors, inoculated with the zinc-deprived sludge, were operated to study restoration of the zinc limitation by zinc supplementation to the bioreactor influent. In a first reactor, no changes to the operational conditions were made. This resulted in methanol accumulation in the reactor effluent after 12 days of operation, which subsequently induced acetogenic activity 5 days after the methanol accumulation started. Methanogenesis could not be recovered by the continuous addition of 0.5 μM ZnCl2 to the reactor for 13 days. In the second reactor, 0.5 μM ZnCl2 was added from its start-up. Although the reactor stayed 10 days longer methanogenically than the reactor operated without zinc, methanol accumulation was observed in this reactor (up to 1.1 g COD-MeOH L−1) as well. This study shows that zinc limitation can induce failure of methanol fed UASB reactors due to acidification, which cannot be restored by resuming the continuous supply of the deprived metal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Balanced operation of anaerobic biological treatment processes, like the upflow anaerobic sludge bed (UASB) reactor, highly depends on the presence of sufficient trace metals to support the growth of the anaerobic microorganisms [1–3]. Trace metal bioavailability affects the overall functioning of anaerobic treatment systems due to their role in enzymatic activity, membrane stability, nutrient transport and energy conservation in methanogenic biomass [4–7].
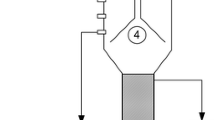
Figure 1 depicts the algorithm how depletion of granular sludge by cobalt [8, 9] or nickel [10, 11] induces methanol accumulation and latter acidification in methanol-fed UASB reactors. Zinc is another key element in methanogenesis (Fig. 2) and its potential deprivation from anaerobic granular sludge has thus far not been investigated. Zinc is involved in coenzyme M activation during the formation of methyl-coenzyme M [4] (Fig. 2). This enzyme system from Methanosarcina barkeri is composed of two methyltransferases, designated MT1 (methanol: 5-hydroxybenzimidazolylcobamide (B12-HBI) methyltransferase) and MT2 (Co-methyl-5-hydroxybenzimidazolylcobamide (CH3-B12-HBI): HS-CoM methyltransferase), which catalyze the formation of methyl-coenzyme M from coenzyme M and methanol [12]. MT1 in the cob(I)amide-reduced form catalyzes methylation with methanol (Fig. 2) [13]. MT2 is a zinc containing monomeric protein which catalyzes methyl transfer from methylated MT1 to coenzyme M (Fig. 2) [14]. The methylation of coenzyme M to methyl-coenzyme M is a reaction in which a thiol group is alkylated, and all enzymes catalyzing alkyl transfers to thiols are zinc-containing proteins [15, 16]. The postulated role of zinc in these enzymes is that of a Lewis acid that activates the thiol groups to be alkylated [17]. In the methanogenic degradation of other substrates, such as dimethylsulfoniopropionate [18] or methylamines [19], enzymatic alkyl transfer reactions are involved as well, which could thus also be affected upon zinc deprivation.
Proposed outline of the mechanism of induction of metal limitation in methanol-fed UASB reactors. 1 Methylotropihic methanogenesis, 2 acetogenesis, 3 acetotrophic methanogenesis. (From [9])
Methyl-coenzyme M formation from methanol and coenzyme M. MT1: methanol: 5-hydroxybenzimidazolylcobamide (B12-HBI) methyl-transferase. MT2: Co-methyl-5-hydroxybenzimidazolylcobamide (CH3-B12-HBI): HS-CoM methyltransferase. (From [4])
The aim of this study is to evaluate the effect of long-term zinc deprivation on the performance of methanol fed UASB reactors and if the outline found for long-term cobalt and nickel deprivation (Fig. 1) also applies to zinc. Moreover, the possible restoration of the activity of the zinc-deprived sludge by continuous zinc addition to the influent was investigated as well. Therefore, the UASB reactor performance was monitored as a function of time. The metabolic properties and possible zinc deficiencies of the sludge that developed in the UASB reactor were characterized by specific methanogenic activity tests. The microbial population dynamics in the sludge granules were observed by fluorescent in situ hybridization (FISH).
Materials and methods
Source of biomass
Methanogenic granular sludge was obtained from a full-scale UASB reactor treating alcohol distillery wastewater at Nedalco (Bergen op Zoom, The Netherlands). The total suspended solids (TSS) and volatile suspended solids (VSS) concentration of the wet sludge were 5.23 (±0.02)% and 4.93 (±0.03)%, respectively.
Feed composition
The reactors were fed a basal medium consisting of methanol, macronutrients and a trace metal solution (Table 1) dissolved in demineralised water. The inorganic macronutrients contained (in milligrams per litre of basal medium): NH4Cl (280), K2HPO4 (250), MgSO4·7H2O (100) and CaCl2·2H2O (10). To ensure pH stability around 7.0, 2.52 g (30 mM) of NaHCO3 was added per litre of basal medium. To avoid precipitation in the storage vessels, the influent was composed of 3 streams: (1) macronutrients and trace metal solution without K2HPO4 and NaHCO3, (2) methanol with NaHCO3 and K2HPO4 and (3) dilution water. Demineralised water was used to prepare the influent and as dilution water.
UASB reactor operation
The experiments were performed using 3.25 L glass cylindrical UASB reactors as described by Fermoso et al. [10]. The reactors were operated in a temperature-controlled room at 30 (±2) °C. The reactors were operated at a hydraulic retention time (HRT) of 12 h and a superficial liquid upflow velocity of 0.35 m h−1. For the influent and recycle flow, peristaltic pumps (type 505S, Watson and Marlow, Falmouth, UK) were used. The produced biogas was led through a water lock filled with a concentrated NaOH (15%) solution in order to remove CO2 and H2S. The volume of produced methane was measured with a wet gas meter (Schlumberger Industries, Dordrecht, The Netherlands).
Experimental design
A UASB reactor (R0) was run during 141 days without zinc in the influent. R0 was inoculated with 1 L of wet sludge. R0 contained an initial biomass concentration of 6.38 g VSS L −1Reactor and was operated at an organic loading rate (OLR) of 8 g COD L−1 day−1, corresponding to a specific loading rate (SLR) of 1.25 g COD VSS−1 day−1 based on the initial VSS concentration. On day 22, the OLR was increased from 8 to 13 g COD L−1 day−1. Sludge growth was excessive and the sludge bed height increased by 80% during the 141 days of operation.
The biomass present in R0 after the 141 days of operation was split in two UASB reactors, both inoculated with 0.9 L wet sludge from R0, corresponding to an initial biomass concentration of 6.89 g VSS L −1Reactor . Both reactors were operated at an OLR of 6.5 g COD L−1 day−1 (SLR of 0.94 g COD g VSS−1 day−1 based on initial VSS concentration). One UASB reactor (R1) was fed without zinc during 31 days and with 0.5 μM zinc in the influent from day 31 till the end of the experiment (day 44). The second UASB reactor (R2) was fed with zinc in the influent from the start: 0.5 μM zinc during 31 days and 5 μM zinc from day 31 till the end of the experiment (day 44).
Specific methanogenic activity test
The effect of different metal concentrations in test medium and the possible zinc limitation of the sludge that developed in the reactor were investigated by specific methanogenic activity (SMA) tests. The SMA of the sludge was determined in duplicate at 30 (±2) °C using on-line gas production measurements as described by Zandvoort et al. [20]. Approximately 1 g (wet weight) of granular sludge was transferred to 120 ml serum bottles containing 50 ml of basal medium with the same composition as the reactor basal medium, supplemented with methanol (4 g COD L−1), acetate (2 g COD L−1) or hydrogen (1 g COD L−1) as the substrate. Data were plotted in a rate versus time curve, using moving average trend lines with an interval of 15 data points.
The SMA with methanol as the substrate was determined on days 50, 69, 91, 111, and 137 for R0, and days 10, 18 and 44 for R1 and R2. The SMA with acetate as the substrate was determined on days 94 and 113 for R0, day 10 for R1 and day 18 for R2. The SMA with hydrogen was determined on day 113 for R0. The samples were always taken from the same place (the mid-height) in the sludge bed.
FISH
Anaerobic granules from operation days 22, 50, 91, 115, 130 and 135 of R0; 10, 18, 30 and 44 of R1 and 10, 18, 30 and 44 of R2 were fixed in 4% (w/v) paraformaldehyde in phosphate buffered saline (PBS; 130 mmol L−1 sodium chloride and 10 mmol L−1 sodium phosphate [pH 7.2]). Cross-sections (thickness, 16 μm; Ø, 0.5–1.5 mm) were prepared as described previously by Sekiguchi et al. [21]. FISH was performed as described by Schramm et al. [22] using Cy5 and Cy3-labeled 16S rRNA-targeted oligonucleotide probes (Biomers.net, Germany): Eub338 [23], Eury498 [24] and Sarci551 [25], specific for the kingdom Eubacteria and Euryarchaeota and Methanosarcina genus, respectively. Hybridization stringencies in the hybridization buffer used were achieved by adding formamide. The Non338 probe [26] was used as a negative control. Specimens were viewed using a Nikon E600 epifluorescent microscope equipped with a 100 W mercury bulb and a Cy3 and Cy5 filter set. The abundance of Eubacteria, Euryarchaeota and Methanosarcina was determined as a fraction of the total area of respective granule sections by image analysis with ImageJ software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://www.rsb.info.nih.gov/ij/, 1997–2007).
Metal composition of the sludge
The metal composition of the sludge was measured on days 22, 50, 81, 107 and 141 for R0; days 10, 18, 31 and 40 for R1 and 10, 18 and 40 for R2. The metal composition of the sludge was determined after destruction with Aqua regia (mixture of 2.5 ml 65% HNO3 and 7.5 ml 37% HCl) as described by Fermoso et al. [10].
Chemical analyses
Methanol and volatile fatty acids (VFA) were determined using gas liquid chromatography as described by Fermoso et al. [9]. Total dissolved metal concentrations in the influent and effluent were determined by ICP-OES (Varian, Australia) in samples acidified with 0.1 M HNO3. The samples were centrifuged at 10,000 rpm to remove particles from the liquid. The total suspended solids (TSS) and volatile suspended solids (VSS) concentrations were determined according to Standard Methods [27]. All chemicals were of analytical or biological grade and purchased from E. Merck AG (Darmstadt, Germany).
Results
Reactor operation
After a start-up period of 10 days, methanol was almost fully converted to methane during the 141 days of R0 operation, despite some incidental VFA accumulation in the effluent on day 45, 76 and 135. VFA accumulated maximally to 0.9 g COD-VFA L−1 and remained at this level not longer than a couple of days (Fig. 3). On day 22, the OLR was increased from 8 to 13 g COD L−1 day−1 without accumulation of methanol or VFA in the influent. The pH was constant (6.86 ± 0.09) during the entire run (Fig. 3). The sludge bed height increased by 80% during the 141 days of operation.
The methanol effluent concentration of R1 started to accumulate on day 10, reaching a maximum value of 3.9 g COD-MeOH L−1 on day 19, corresponding to 65% of the influent methanol concentration (Fig. 4). On day 16, VFA started to accumulate and the pH of the R1 effluent dropped from 6.8 to 6.0. The VFA concentration reached 1.8 g COD-VFA L−1 on day 19 and no methane was produced anymore. The OLR was decreased to 2.5 g COD L−1 day−1 on day 19, resulting in a decrease in the methanol effluent concentration of R1 up to 0.5 g COD-MeOH L−1. However, the R1 effluent VFA concentration was stable at 1.8 g COD-VFA L−1. On day 31, 0.5 μM of zinc was included in the R1 influent. The methanol accumulation gradually decreased up to 0.14 g COD-MeOH L−1. However, the methane production did not recover and all supplied methanol was converted to VFA (mainly acetate) at the end of the experiment (day 44).
Complete methanol removal was observed during 16 days of R2 operation (Fig. 5). On day 16, the methanol effluent concentration started to accumulate to 0.3 g COD-MeOH L−1 and on day 18, VFA started to accumulate as well. On day 19, R2 effluent methanol and VFA concentrations reached 1.1 g COD-MeOH L−1 and 1.1 g COD-VFA L−1, respectively, and only 62% of the supplied methanol was converted to methane. The pH of the R2 effluent dropped from 6.8 (day 16) to 6.2 (day 19). The OLR was therefore decreased to 2.5 g COD L−1 day−1 on day 19, resulting in a gradual decrease in methanol accumulation to 0.09 g COD-MeOH L−1 on day 23. However, the R2 effluent VFA concentration increased up to 1.6 g COD-VFA L−1 on day 30 and only 15% of the fed methanol was converted to methane on that day. On day 31, the zinc concentration in the R2 influent was increased to 5 μM. This did not induce significant changes in the R2 effluent methanol or VFA concentration within the time span investigated (day 31–44).
Evolution of the SMA over time
Induction of zinc limitation was observed in R0 sludge after 50–70 days of operation (Table 2; Fig 6a). On day 50, the SMA with methanol as the substrate in a medium with no metals was 14% lower than in a medium with zinc solely. Similarly, the SMA with methanol as the substrate in a medium with no metals on day 69 was 18 and 21% lower than in a medium with zinc solely and in a medium with the complete metal solution except zinc, respectively. It should be noted that on day 69, the SMA on methanol had increased for all the different trace metal solutions tested compared to day 50.
On day 90, a further increase in SMA of the R0 sludge with methanol as the substrate was observed (Table 2), but no metal limitation of the SMA on methanol was observed on day 90 as the tests with zinc and without zinc had a similar SMA (Table 2). On day 111, the SMA on methanol increased again for all the trace metal solutions tested and metal limitation was observed again. The SMA on methanol with the complete metal solution was 7, 13 and 24% higher than in, respectively, a medium with no metal, a medium with the complete metal solution except zinc and a medium with zinc solely. The SMA with acetate and hydrogen as the substrates, measured on day 111, were 0.51 g CH4-COD g VSS−1 day−1 and 0.82 g CH4-COD g VSS−1 day−1, respectively. The latter values indicate that maximally 38% of the total amount of methane formed could be produced via acetate and/or hydrogen as the intermediates if they are compared with the SMA on methanol on the same day, 3.49 g CH4-COD g VSS−1 day−1 (Table 2).
On day 137, a clear zinc limitation was observed (Table 2; Fig 6a): the SMA on methanol with the complete metal solution except zinc or in a medium with no metals at all was 24 and 28% lower than the SMA on methanol in the medium with the complete metal solution. The SMA on methanol with the complete metal solution was slightly higher (3%) than in a medium with zinc solely.
After 10 days of operation of R1, at the time that methanol accumulation was observed, the SMA on methanol had decreased to 2.26 g CH4-COD g VSS−1 day−1 in a medium containing the complete metal solution except zinc, corresponding to a 52% lower SMA than the R0 sludge had on day 137 for the same medium (Table 2; Fig 6b). The SMA of the R1 sludge with acetate as the substrate on day 10 (0.31 g CH4-COD g VSS−1 day−1) indicates that maximally 13% of the methane formed could be produced via acetate as an intermediate (Table 2). On day 18, when VFA accumulation was observed, the SMA with methanol as the substrate had decreased to 0.96 g CH4-COD g VSS−1 day−1 in a medium containing the complete trace metal solution except zinc. On day 44, 13 days after zinc addition (0.5 μM) to the R1 influent, the SMA with methanol as the substrate had decreased to as low as 0.17 g CH4-COD g VSS−1 day−1 in a medium containing the complete trace metal solution except zinc (Table 2).
After 10 days of operation of R2, the SMA of the R2 sludge on methanol was similar to the SMA observed of R0 sludge on day 137. Interestingly, no differences in SMA with methanol as the substrate were observed with the different metal solutions used (Table 2; Fig 6c). On day 18, when methanol and VFA accumulated, the SMA had decreased to 1.93 g CH4-COD g VSS−1 day−1 in a medium containing the complete trace metal solution except zinc. A low SMA of the R2 sludge with acetate as the substrate was observed at this day (0.35 g CH4-COD g VSS−1 day−1). On day 44, 13 days after the zinc increase in the R2 influent to 5 μM, the SMA of the R2 sludge had decreased to 1.52 g CH4-COD g VSS−1 day−1 in a medium containing the complete trace metal solution except zinc (Table 2).
Metal dynamics
Zinc was lost from the granular sludge at a rate of 10 μg g TSS−1 day−1 during the first 80 days of R0 operation (Fig. 7a). In the same period, zinc was washed out from the reactor via the effluent at a rate of 0.48 μg L−1 day−1. Nickel and iron concentrations in the granular sludge decreased at a rate of 1.3 and 106 μg g TSS−1 day−1, respectively (Fig. 7c, e). The cobalt concentration in the R0 sludge was, however, constant during the first 80 days (0.032 ± 0.005 μg g TSS−1) (Fig. 7c). Cobalt and nickel showed a distinct difference in retention, even when they were dosed at the same concentration (Table 1) during the same period. From day 80 to the end of R0 operation (day 141), the zinc, cobalt, nickel and iron concentration in the granular sludge were constant: 0.18 (±0.04), 0.040 (±0.07), 0.06 (±0.01) and 1.7 (±0.5) μg g TSS−1, respectively (Fig. 7a, c, e).
Evolution of the trace metal and sulfur concentration in the sludge over time. a Zinc concentration in the sludge in R0, b zinc concentration in the sludge in R1 (triangle) and R2 (square), c cobalt (triangle) and nickel (square) sludge concentration in R0, d cobalt (triangle) and nickel (circle) sludge concentration in R1 and cobalt (square) and nickel (diamond) sludge concentration in R2, e iron sludge concentration in R0. f Iron sludge concentration in R1 (square) and R2 (triangle)
The zinc concentration of the R1 sludge on day 19 was about half compared to day 13: 0.030 and 0.058 μg g TSS−1, respectively (Fig. 7b). The decrease in the zinc concentration of the R1 sludge coincides with the pH drop from 6.8 to 6.0 of the R1 mixed liquor (Fig. 4c). Zinc addition (5 μM) to R1 influent from day 32 onwards did not increase the zinc concentration of the granular sludge (Fig. 7b). In the same period, the cobalt and nickel concentration almost doubled compared to the concentration in the R1 sludge prior to the addition (Fig. 7d).
After 31 days of zinc addition to R2, the zinc concentration in the R2 sludge had almost doubled compared to the seed sludge used to inoculate R2 (Fig. 7b). The zinc concentration of the R2 sludge had not increased on day 40, 9 days after the increase of the zinc concentration in the influent from 0.5 to 5 μM. During the 44 days of R2 operation, the cobalt and iron concentration in the R2 sludge were stable at 0.056 (±0.003) μg g TSS−1 and 1.15 (±0.1) μg g TSS−1, respectively (Fig. 7d, f). In the same period, the nickel concentration in the R2 sludge increased from 63 μg g TSS−1 on day 10 to 158 μg g TSS−1 on day 40 (Fig. 7d).
Evolution of key microbial population
The abundance of the Euryarchaeota and Methanosarcina in the R0 granules was stable during the 141 operation days (Fig. 8a, c, d, f). The abundance of the Eubacteria followed a slight decreasing trend. On day 130, the abundance of cells hybridizing to Eub338 had decreased compared to day 50 (Fig. 8b, e). On day 135, an increase of cells hybridizing to Eub338 was observed, which coincides with a short VFA accumulation in the effluent (Fig. 3a vs. Fig. 8b, e).
Genus-specific hybridizations with the Eury498 and Eub338 probe revealed a temporal evolution of the Euryarchaeota and Eubacteria populations in the R1 run. Firstly, Eury498-positive cells were abundant around the periphery of respective sections on day 10 (Fig. 9a). Eub338-positive cells indicated very few Eubacteria cells, which were mainly present in infrequent clusters around the edge of the granule sections on day 10 (Fig. 9b). On day 18, the Euryarchaeota and Eubacteria populations were located throughout the sections of the sludge. An increase in Eubacteria population was observed on day 18. By day 30, the relative abundance of the Euryarchaeota was reduced in R1 sludge, which were present in relatively smaller, infrequent clusters around the edge of the granule sections (Fig. 9a). However, the Eubacteria population on day 30 (Fig. 9e) was as abundant as on day 18. On day 44, after zinc addition in R1, a further reduction in the Euryarchaeota population was observed (Fig. 9d). The Eub338-positive cells were typically observed in densely packed clusters on day 44, but they were less dense than on day 30 (Fig. 9e). The abundance of the Methanosarcina followed a slight decreasing trend. On day 30, the abundance of cells hybridizing to Sarci551 had decreased compared to day 10 (Fig. 9c). A further reduction was evident by day 44 (Fig. 9f).
The Euryarchaeota and Methanosarcina populations were dynamic in the R2 granules. Firstly, Eury498-positive cells were observed in densely packed clusters in the centre of the granule section on day 10 (Fig. 10a). Sarci551-positive cells were present in clusters around the edge of the granule sections on day 10 (Fig. 10c). By day 18, an increased abundance of Eury498-positive clusters was detected. The Methanosarcina population was observed in less dense clusters than on day 10. On day 30, a slight reduction in the number of Euryarchaeota was observed. A slight increase of Sarci551-positive cells was evident by day 30, which were more dispersed than on day 18. On day 44, after the zinc concentration was increased in R2 influent, a reduction in the Methanosarcina population size was observed (Fig. 10f). More Eury498-positive dispersed cells were observed on day 44 (Fig. 10d) than on day 30. Eub338-positive cells in R2 were abundant around the periphery of respective sections on day 10 (Fig. 10b). On day 18, a decrease in abundance of cells hybridizing to Eub338 compared to day 10 was observed (Fig. 10b). No clear further reduction was observed by days 30 and 44 (Fig 10e).
Discussion
Methanogenesis under zinc limiting conditions
This study shows that omission of zinc from the feed of methanol-fed anaerobic granular sludge bioreactors leads to zinc limitation of the granular sludge (Fig. 6a; Table 2), which reduces the methanol removal efficiency (Fig. 4a), and ultimately leads to acidification of the UASB reactor (Fig. 4a, c). Zinc depletion thus leads to a drop in the SMA with methanol as the substrate according to the algorithm present in Fig. 1, similar to the way cobalt [9, 20] and nickel [10] deprivation of UASB reactors occurs.
Table 3 shows that the zinc limitation is less pronounced compared to cobalt or nickel limitation of methanol fed UASB reactors under similar conditions. The rather long time period needed to achieve zinc limitation (>130 days) was also required to achieve nickel limitation [10] (Table 3). This is, in contrast, much longer compared to the time required for cobalt limitation, which can already develop after 2 days of operation at an OLR of 4.5 g COD L−1 day−1 [9] (Table 3). This clearly reflects the biochemical role of cobalt in the direct methanogenesis with methanol as the substrate, e.g. the central ion of cobalamine is a cobalt ion [28]. In contrast, both zinc and nickel play only an indirect role in the functioning of enzymes of methanogenesis, such as methyl-coenzyme M reductase for nickel [29] or coenzyme M methyltransferase for zinc [4].
The omission of zinc from the UASB reactor influent had some unexpected effects. The maximal SMA achieved in the present study was much higher (3.49 g CH4-COD g VSS−1 day−1) than the maximal SMA achieved in UASB reactors from which nickel or cobalt is omitted from the influent: 1.67 g CH4-COD g VSS−1 day−1 [10] and 0.45 g CH4-COD g VSS−1 day−1 [9], respectively (Table 3). Inhibition by zinc could be one of the reasons for the lower activity of sludge developing under cobalt or nickel limitation (but in the presence of zinc). Tan et al. [30] showed that zinc could replace nickel in Acetyl Coenzyme A synthase and subsequently inactivate this enzyme, involved in acetogenesis from methanol. Moreover, the sludge bed growth was much higher compared to that in cobalt or nickel deprived reactors (Table 3). This might be due to the higher methanogenic activity of the sludge present in the zinc-limited reactor or that the presence of zinc in the cobalt and nickel limited reactors had a growth retarding effect [31].
Only a few papers have so far reported on zinc deprivation during methanogenesis. Osuna et al. [32] studied metal deprivation in VFA fed UASB reactors. The latter authors found that when adding solely zinc to the medium, the SMA with acetate as the substrate of metal deprived sludge increased by 36%. Sauer and Thauer [33] found in enzymatic studies that the transfer of the methyl group from CH3-MT1 to coenzyme M by MT2 depends on the Zn2+ concentration. The Km value of this transfer reaction amounted to 0.25 nM free Zn2+. This Km value is around 240 times lower than the dissolved zinc concentration in the medium of the reactor (60 nM) at the moment the limitation was developed in the present study. This difference between the Km value found by the latter authors (with enzymes) and the dissolved zinc concentration at the time of limitation in the present study (with sludge granules) can be explained by several interactions between the metal cation and the heterogeneous and complex environment inside a UASB reactor. Complexation by bicarbonates, phosphates, soluble sulfides or soluble extrapolymeric substance produced by the biomass reduce the free metal concentration [34].
The dissolved metal concentrations can be only 0.5–4% of the total metal concentration in anaerobic environments [32], since metal speciation under these conditions depends on the concentration of the metal, its redox state and competition with other metals for adsorption and complexation sites. Assuming the Km value for the zinc requirement of Methanosarcina cells is in the range reported by Sauer and Thauer [33] and the maximum dissolved zinc concentration is 4% of the total zinc concentration [32], the required free zinc fraction would be only around 0.016% of the total zinc concentration present. As the reactor contains already a substantial zinc pool via the inoculum sludge (Fig. 7a), dosing zinc to bioreactors will not always be necessary to support the zinc requirement of the methanogens. A slight change in the speciation of zinc, e.g. by a change in redox or by exposure to an electrokinetic field [35], can release the zinc present into a bioavailable pool, and thus support the zinc dependent enzymatic processes.
Acetogenesis under zinc limiting conditions
Due to the SMA drop with methanol as the substrate, methanol started to accumulate in R1 (Fig. 4) and in R2 (Fig. 5). Methanol accumulation was followed by acetate accumulation, similar to the outline shown in Fig. 1. Once the methanol accumulation surpasses a threshold value, about 50 mM in R1 and 20 mM in R2, acetogens outcompeted methanogens for methanol, due to their higher affinity for methanol at high methanol concentrations [36]. The threshold methanol concentration of cobalt and nickel deprived reactors to get acidification were in the same range as observed in this study with zinc deprived UASB reactors (Table 3). The similar threshold value for acidification found for methanol fed UASB reactors limited by different metal (Table 3) indicates that enhancement of acetogens upon exceeding the methanol threshold value is metal independant.
Effect of continuous zinc addition
The continuous zinc addition to R2, prior to methanol or acetate accumulation (Fig. 5a), supported methanogenesis during a longer period than in R1 to which initially no zinc was added (Fig. 4a), but could not prevent its acidification. This suggests that the amount of zinc added to the R2 influent was not enough to support stable high rate methylotrophic and acetoclastic methanogenesis. This contrasts the almost double zinc concentration of the R2 sludge (Fig. 7b). It is expected that the most important process for zinc accumulation in the sludge is zinc sulfide precipitation. The low solubility product of zinc sulfide results in extremely low free zinc ion concentrations [37], which might be insufficient to support the trace metal requirement of the methanogens. Therefore, further investigations about dosing methods need to take into account the speciation that involves zinc upon its addition in anaerobic environments [38] and the high wash out rates in UASB reactors [39].
The Methanosarcina and Euryarchaeota populations in R2 were observed in dense clusters in the centre of the granule but in infrequent clusters in the periphery of the granule in R1 after 10 days of operation, when 0.5 μM zinc was continuously added to the influent of the R2 reactor but no zinc to the R1 influent. This suggests that the zinc addition stimulated the growth of methanogens in clusters. Indeed, as shown by other authors [9, 10, 21], the association of methanogens in clusters seems to be correlated with the development of the methanogenic population.
Addition of zinc to the reactor influent after methanol and acetate accumulation in the reactors sustained or stimulated acetate accumulation in both reactors (Figs. 4a, 5a). Methylotrophic or acetoclastic methanogenesis did not recover by the continuous zinc addition to R1 (Table 2). In the case of R2 sludge, a five times higher SMA on methanol with zinc in the medium compared to the SMA on methanol in the absence of metals on day 44 (Fig. 6c) indicates that the recovery of the methanogenesis by means of zinc addition should be possible. However, the SMA increase on methanol achieved in R2 upon continuous zinc dosing was not high enough to avoid methanol accumulation in the effluent (Fig. 5a), which induces reactor acidification (Fig. 5c). Both observations for R1 and R2 are in agreement with the FISH pictures, that indicated a further decrease of the Euryarcheaota and Methanosarcina population after the zinc addition to R1 (Fig. 9f) and R2 (Fig. 10f) after 31 days of operation.
The same enhancement of acetogenesis and a similar decrease of the Methanosacina cell number was observed by Fermoso et al. [9] upon cobalt dosing to an acidified cobalt limited methanol fed UASB reactor at pH 7.0. In contrast, in a nickel limited methanol fed UASB reactor with strong acetate accumulation at pH 6.9, the continuous nickel addition enhanced methylotrophic methanogenesis and acidification disappeared within 2 days [10]. The different behavior upon nickel addition might be due to the lower zinc dependence of the enzymatic system of methanogenesis (Fig. 2) compared to nickel [4]. In the case of cobalt, the high cobalt dependence [40], but slight nickel dependence [41], of the enzymatic system of acetogens, most probably hampered the restoration of fully methanogenic reactor.
References
Oleszkiewicz JA, Sharma VK (1990) Stimulation and inhibition of anaerobic processes by heavy-metals—a review. Biol Waste 31(1):45–67
Singh RP, Kumar S, Ojha CSP (1999) Nutrient requirement for UASB process: a review. Biochem Eng J 3(1):35
Jefferson B, Burgess JE, Pichon A, Harkness J, Judd SJ (2001) Nutrient addition to enhance biological treatment of greywater. Water Res 35(11):2702
Sauer K, Thauer RK (2000) Methyl-coenzyme M formation in Methanogenic archaea: involvement of zinc in coenzyme M activation. Eur J Biochem 267(9):2498–2504
Whitman WB, Ankwanda E, Wolfe RS (1982) Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol 149(3):852–863
Mulrooney SB, Hausinger RP (2003) Nickel uptake and utilization by microorganisms. FEMS Microbiol Rev 27(2–3):239–261
Gavel OY, Bursakov SA, Calvete JJ, George GN, Moura JJG, Moura I (1998) ATP sulfurylases from sulfate-reducing bacteria of the genus Desulfovibrio. A novel metalloprotein containing cobalt and zinc. Biochemistry 37(46):16225–16232
Zandvoort MH, van Hullebusch ED, Golubnic S, Gieteling J, Lens PNL (2006) Induction of cobalt limitation in methanol-fed UASB reactors. J Chem Technol Biotechnol 81(9):1486–1495
Fermoso FG, Collins G, Bartacek J, O’Flaherty V, Lens P (2008) Acidification of methanol-fed anaerobic granular sludge bioreactors by cobalt deprivation: induction and microbial community dynamics. Biotechnol Bioeng 99:49–58
Fermoso FG, Collins G, Bartacek J, O’Flaherty V, Lens P (2008) Role of nickel in high rate methanol degradation in anaerobic granular sludge bioreactors. Biodegradation. doi:10.1007/s10532-008-9177-3
Zandvoort MH, Osuna MB, Geerts R, Lettinga G, Lens P (2002) Effect of nickel deprivation on methanol degradation in a methanogenic granular sludge reactor. J Ind Microbiol Biotechnol 29:268–274
van der Meijden P, Jansen LPJM, van der Drift C, Vogels GD (1983) Involvement of corrinoids in the methylation of coenzyme M (2-mercaptoethanesulfonic acid) by methanol and enzymes from Methanosarcina barkeri. FEMS Microbiol Lett 19(2–3):247
Daas PPJH, Hagen WR, Keltjens JT, van der Drift C, vogels GD, (1996) Activation mechanism of methanol: 5-hydroxybenz-imidazolylcobamide methyltransferase from Methanosarcina barkeri. J Biol Chem 271:22346–22351
Harms U, Thauer RK (1996) Methylcobalamin: coenzyme M methyltransferase isoenzymes MtaA and MtbA from Methanosarcina barkeri. Cloning, sequencing and differential transcription of the encoding genes, and functional overexpression of the mtaA gene in Escherichia coli. Eur J Biochem 235(3):653–659
Matthews RG, Goulding CW (1997) Enzyme-catalyzed methyl transfers to thiols: the role of zinc. Curr Opin Chem Biol 1(3):332
Penner-Hahn J (2007) Zinc-promoted alkyl transfer: a new role for zinc. Curr Opin Chem Biol 11(2):166
Goulding CW, Matthews RG (1997) Cobalamin-dependent methionine synthase from Escherichia coli: involvement of zinc in homocysteine activation. Biochemistry 36(50):15749–15757
van der Maarel MJEC, Hansen TA (1997) Dimethylsulfoniopropionate in anoxic intertidal sediments: a precursor of methanogenesis via dimethyl sulfide, methanethiol, and methiolpropionate. Mar Geol 137(1–2):5
Zhilina TN, Zavarzin GA (1990) Extremely halophilic, methylotrophic, anaerobic bacteria. FEMS Microbiol Lett 87(3–4):315
Zandvoort MH, Geerts R, Lettinga G, Lens PNL (2002) Effect of long-term cobalt deprivation on methanol degradation in a methanogenic granular sludge bioreactor. Biotechnol Prog 18(6):1233
Sekiguchi Y, Kamagata Y, Nakamura K, Ohashi A, Harada H (1999) Fluorescence in situ hybridization using 16S rRNA-targeted oligonucleotides reveals localization of methanogens and selected uncultured bacteria in mesophilic and thermophilic sludge granules. Appl Environ Microbiol 65(3):1280–1288
Schramm A, de Beer D, Wagner M, Amann R (1998) Identification and activities in situ of Nitrosospira and Nitrospira spp. as dominant populations in a nitrifying fluidized bed reactor. Appl Environ Microbiol 64(9):3480–3485
Stahl DA, Amann R (1991) Development and application of nucleic acid probes. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 205–248
Burggraf S, Mayer T, Amann R, Schadhauser S, Woese CR, Stetter KO (1994) Identifying members of the domain archaea with ribosomal-RNA-targeted oligonucleotide probes. Appl Environ Microbiol 60(9):3112–3119
Sorensen AH, Torsvik VL, Torsvik T, Poulsen LK, Ahring BK (1997) Whole-cell hybridization of Methanosarcina cells with two new oligonucleotide probes. Appl Environ Microbiol 63(8):3043–3050
Wallner G, Amann R, Beisker W (1993) Optimizing fluorescent insitu hybridization with ribosomal-RNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14(2):136–143
APHA/AWWA (1998) Standard methods for the examination of water and wastewater. 20th edn, USA
Florencio L, Field JA, Lettinga G (1994) Importance of cobalt for individual trophic groups in an anaerobic methanol-degrading consortium. Appl Environ Microbiol 60(1):227–234
Ellefson WL, Whitman WB, Wolfe RS (1982) Nickel containing factor F430: chromophore of the methylreductase of Methanobacterium. Proc Natl Acad Sci USA 79:3707–3710
Tan X, Bramlett MR, Lindahl PA (2004) Effect of Zn on acetyl coenzyme A synthase: evidence for a conformational change in the subunit during catalysis. J Am Chem Soc 126(19):5954–5955
Salminen J, Tan Anh B, van Gestel CAM (2001) Indirect effects of zinc on soil microbes via a keystone enchytraeid species. Environ Toxicol Chem 20(6):1167–1174
Osuna MB, Zandvoort MH, Iza JM, Lettinga G, Lens PNL (2002) Effects of trace element addition on volatile fatty acid conversion in anaerobic granular sludge reactors. Environ Technol 24:557–587
Sauer K, Thauer RK (1997) Methanol:coenzyme M methyltransferase from Methanosarcina barkeri. Zinc dependence and thermodynamics of the methanol:cob(I)alamin methyltransferase reaction. Eur J Biochem 249(1):280–285
van Hullebusch ED, Zandvoort MH, Lens PNL (2003) Metal immobilisation by biofilms: mechanisms and analytical tools. Rev Environ Sci Biotechnol 2(1):9
Virkutyte J, van Hullebusch E, Sillanpaa M, Lens P (2005) Copper and trace element fractionation in electrokinetically treated methanogenic anaerobic granular sludge. Environ Pollut 138(3):517
Florencio L, Field JA, Lettinga G (1995) Substrate competition between methanogens and acetogens during the degradation of methanol in UASB reactors. Water Res 29(3):915
Luther GW, Rickard DT, Theberge S, Olroyd A (1996) Determination of metal (Bi)sulfide stability constants of Mn2+, Fe2+, Co2+, Ni2+, Cu2+, and Zn2+ by voltammetric methods. Environ Sci Technol 30(2):671–679
Webb SMS, Gaillard J-F, Nolan MA, Jackson BE, Stahl DA (2000) In an EXAFS study of zinc speciation in anaerobic microbes, Preprints of Extended Abstracts presented at the ACS National Meeting, American Chemical Society, Division of Environmental Chemistry; 2000; pp 326–328
Zeng Y, Mu SJ, Lou SJ, Tartakovsky B, Guiot SR, Wu P (2005) Hydraulic modeling and axial dispersion analysis of UASB reactor. Biochem Eng J 25(2):113
Bainotti AE, Nishio N (2000) Growth kinetics of Acetobacterium sp. on methanol-formate in continuous culture. J Appl Microbiol 88(2):191–201
Diekert G, Konheiser U, Piechulla K, Thauer RK (1981) Nickel requirement and factor F430 content of methanogenic bacteria. J Bacteriol 148(2):459–464
Acknowledgments
This research was funded by the Marie Curie Excellence Grant “Novel biogeological engineering processes for heavy metal removal and recovery” (MEXT-CT-2003-509567).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Fermoso, F.G., Collins, G., Bartacek, J. et al. Zinc deprivation of methanol fed anaerobic granular sludge bioreactors. J Ind Microbiol Biotechnol 35, 543–557 (2008). https://doi.org/10.1007/s10295-008-0315-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0315-z