Abstract
The study aims to evaluate multiparametric magnetic resonance imaging (MRI) for differentiating Follicular thyroid neoplasm (FTN) from non-FTN and malignant FTN (MFTN) from benign FTN (BFTN). We retrospectively analyzed 702 postoperatively confirmed thyroid nodules, and divided them into training (n = 482) and validation (n = 220) cohorts. The 133 FTNs were further split into BFTN (n = 116) and MFTN (n = 17) groups. Employing univariate and multivariate logistic regression, we identified independent predictors of FTN and MFTN, and subsequently develop a nomogram for FTN and a risk score system (RSS) for MFTN prediction. We assessed performance of nomogram through its discrimination, calibration, and clinical utility. The diagnostic performance of the RSS for MFTN was further compared with the performance of the Thyroid Imaging Reporting and Data System (TIRADS). The nomogram, integrating independent predictors, demonstrated robust discrimination and calibration in differentiating FTN from non-FTN in both training cohort (AUC = 0.947, Hosmer-Lemeshow P = 0.698) and validation cohort (AUC = 0.927, Hosmer-Lemeshow P = 0.088). Key risk factors for differentiating MFTN from BFTN included tumor size, restricted diffusion, and cystic degeneration. The AUC of the RSS for MFTN prediction was 0.902 (95% CI 0.798–0.971), outperforming five TIRADS with a sensitivity of 73.3%, specificity of 95.1%, accuracy of 92.4%, and positive and negative predictive values of 68.8% and 96.1%, respectively, at the optimal cutoff. MRI-based models demonstrate excellent diagnostic performance for preoperative predicting of FTN and MFTN, potentially guiding clinicians in optimizing therapeutic decision-making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Follicular thyroid neoplasm (FTN), primarily driven by mutations in the Rat sarcoma (RAS) gene family, encompasses a range of thyroid tumors– follicular thyroid adenoma (FTA), follicular tumors with uncertain malignant potential (FT-UMP), and follicular thyroid carcinoma (FTC) [1, 2]. Malignant follicular thyroid neoplasm (MFTN) often leads to a worse prognosis, such as local recurrence, distant metastases, and reduced overall survival [3, 4]. Thus, accurately diagnosing malignancy of FTN is crucial for informed clinical decision-making. For patients with thyroid nodules, ultrasonography serves as the primary method for assessing malignancy risk and guiding fine-needle aspiration (FNA) decisions. However, ultrasonography and biopsy frequently fall short in differentiating FTA from FTC [5]. Several studies have sought to identify ultrasonography features that could differentiate FTA and FTC, but results remain inconsistent [6, 7]. FNA, challenged by overlapping cytological features of FTC and FTA, often fails to provide a clear distinction [8, 9]. Therefore, there is a pressing need for an accurate, noninvasive, and preoperative prediction model for MFTN.
Distinguishing benign FTN (BFTN) from MFTN fundamentally requires differentiating FTN from non-FTN. Various Thyroid Imaging Reporting and Data System (TIRADS) based on ultrasonography risk factors, including the American College of Radiology TIRADS (ACR-TIRADS) [10], Korean TIRADS (K-TIRADS) [11], European TIRADS (EU-TIRADS) [12], Kwak-TIRADS [13], and Chinese-TIRADS (C-TIRADS) [14] are widely employed to manage thyroid nodules effectively. However, these systems are primarily established using malignant ultrasonography features of papillary thyroid carcinoma (PTC) rather than FTC [15, 16]. As the existing TIRADS classifications have shown ineffective in accurately identifying FTNs, Li et al. [17] proposed the need for a more effective risk stratification system. Lin et al. [16] highlighted the inefficiency of different TIRADS in managing patients with FTN, with 65.3–93.1% unnecessary biopsy rates, underscoring the need for FTN-specific stratification system. According to the American Thyroid Association guidelines, diagnostic surgical resection remains the standard treatment [18]; This approach predominately results in thyroidectomy for patients whose FNA cytology suggests a “follicular neoplasm” or “suspicious of follicular neoplasm”. However, it is notable that approximately 60–90% of these cases are benign [19, 20]. Hence, establishing an effective method for distinguishing FTN from non-FTN in thyroid nodules is imperative.
T2-weighted imaging (T2WI), dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), and diffusion-weighted imaging (DWI) anchor the core of multiparametric MRI [21]. Recently, the role of MRI has expanded, increasingly facilitating the preoperative evaluation of thyroid nodules and PTC [22,23,24,25,26]. MRI is recommended for evaluating thyroid nodules classified as TI-RADS category 4 or above, for cases with suspected aggressive thyroid cancer, and for large nodules where there is a need to distinguish between benign or malignant characteristics, evaluate cancer aggressiveness, and understand the nodule’s anatomical relationships with adjacent structures. However, there is a scarcity of research that focuses on multiparametric MRI features specific to patients with FTN. This study aims to evaluate the diagnostic efficacy of multiparametric MRI in distinguishing FTN from non-FTN and predicting FTN malignancy.
Materials and Methods
Patients and Study Design
The Institutional Review Board of our institute approved this study, and informed consent was exempted due to the study’s retrospective nature. We reviewed the medical records of consecutive patients who underwent thyroid nodule surgery from 2017 to 2022 in our institution. The inclusion criteria were the following: (1) enhanced thyroid MRI examination performed within one month before surgery; (2) complete pathology of postoperative specimens. The exclusion criteria were the following: (1) lesion < 5 mm; (2) poor image quality with motion or imaging artifacts that interfered with diagnosis; (3) missing images or postoperative pathological results; (4) bilateral diffuse lesions with different pathological types; (5) patients who underwent FNA or partial thyroidectomy before the MRI examination; (6) patients with unclear pathology; (7) patients with non-enhancing nodules considered a benign sign. Finally, this study included 702 thyroid nodules from 462 patients.
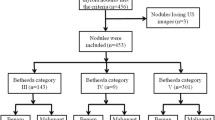
According to the postoperative pathology, lesions were split into FTN group (n = 133) and non-FTN group (n = 569). Seven hundred and two thyroid nodules were randomly divided into training cohort (482 thyroid nodules) and validation cohort (220 thyroid nodules) in a 7:3 ratio. In the same way, FTN was divided into an MFTN group (n = 17) and BFTN group (n = 116) according to postoperative pathology. BFTN included adenomatous goiter and FTA. MFTN included FT-UMP and FTC. Figure 1 shows the study flow diagram in detail.
Magnetic Resonance Imaging Acquisition
MRI examinations were performed with a 1.5T MRI scanner from GE Healthcare (EXCITE HD) equipped with a customized 8-channel neck coil from Chenguang Medical Technology Ltd. The MRI sequences included (1) coronal and axial fat-saturated T2WI, (2) axial T1WI, (3) DWI (single-shot spin-echo echo-planar imaging) at b values of 0, 800–1000 s/mm², and (4) axial multiphasic contrast-enhanced T1WI. Gadolinium contrast medium (Magnevist; Bayer Healthcare, Berlin, Germany) was injected at 0.2 ml/kg and 3 ml/s, followed immediately by a 20-ml physiological saline flush. Images from six phases were acquired at 30 s, 60 s, 120 s, 180 s, 240 s, and 300 s after injection of the contrast agent, and the patients were instructed to hold their breath. Table 1 lists the detailed MRI acquisition parameters.
TIRADS
Two ultrasonography experts (with more than 10 years of experience) who were blinded to the histopathological results performed a retrospective analysis of ultrasonography features of thyroid nodules, and then they arrived at a consensus. The ultrasonography features included composition, echogenicity, margin, shape, echogenic foci, calcification, hypoechoic peripheral halo, extrathyroidal extension, and suspicious cervical lymph node. All thyroid nodules were categorized according to ACR-, K-, EU-, Kwak-, and C-TIRADS. For ACR-TIRADS, K-TIRADS and EU-TIRADS, category ≥ 4 or 5 was considered malignant; for Kwak-TIRADS and C-TIRADS, category ≥ 4b or 4c was considered malignant, respectively. The diagnostic performance of five TIRADS was calculated. Because of a lack of ultrasonography images, 15 FTNs were excluded. Finally, 118 FTNs were assessed based on five different TIRADS.
Magnetic Resonance Imaging Analysis
Two radiologists with five and nine years of thyroid MRI experience independently interpreted the MRI findings using Advantage Workstation 4.5 (GE Healthcare, Waukesha, WI, USA) and picture archiving and communication system (PACS). To ensure a blinded study, the tumor histopathology results were kept from the MRI observers. The final decision was made by consensus if there was disagreement between two radiologists. Tumor size and number (unifocal or multifocal) were assessed (the largest linear dimension of nodules < 4 cm or ≥ 4 cm). The following qualitative MRI features were evaluated: (1) high signal intensity on T2WI, low signal intensity on T2WI, and high signal intensity on T1WI; (2) cystic degeneration; (3) flow-void signal; (4) restricted diffusion; (5) pseudocapsule; (6) reversed halo sign; (7) fissure-filling enhancement; (8) hyperintense on T2WI with enhancement; (9) uniformity of contrast enhancement (homogeneous or heterogeneous); (10) wash-out pattern; 11) hyperenhancement in early phase; 12) change of lesion in multiphasic enhancement. MRI feature definitions and diagrams are detailed in Supplementary materials.
Model Establishment
First, we developed a nomogram for the prediction of FTN in the training cohort. The imaging factors underwent univariate analysis to determine the predictive factors related to the FTN. Subsequently, multivariate logistic regression was utilized to enhance the accuracy and reliability of FTN prediction. We selected the optimal model by Akaike Information Criteria. Models were developed based on the multivariate analysis.
Subsequently, a risk score system (RSS) for distinguishing BFTN and MFTN was constructed. The risk score was generated by utilizing the β coefficient of the logistic regression models for all factors that attained statistical significance in the multivariate analysis. To simplify the calculation process, the decimal was omitted, and the integer part of the β coefficient was taken. The sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of the RSS of MFTN at optimal cutoff value were compared with the same values from the TIRADSs. Using the net reclassification index (NRI), we evaluated the ability of different models to reclassify patients into the MFTN or BFTN group.
Statistical Analysis
All statistical analyses were performed with SPSS Statistics 26.0 (IBM Corp, Armonk, NY, USA) and R software 4.2.0 (http://www.r-project.org). Continuous variables were represented as mean ± standard deviation (SD) or categorical variables as percentages. We compared continuous variables with an independent t-test. Categorical variables were compared by a Chi-square test or Fisher’s exact test. The Cohen’s Kappa test was used to assess the concordance between the two radiologists. By maximizing Youden’s index, the optimal cutoff value of the RSS for predicting MFTN was determined from a receiver operating characteristic (ROC) curve analysis. Bootstrap validation with 1000 resamples was adopted for evaluation of the RSS. Nomogram was constructed by the R software package “rms”. Hosmer-Lemeshow test was used to assess the model’s goodness-of-fit, with P ≥ 0.05 indicating a good fit. We conducted ROCs analyses, calibration curve analyses, and decision curve analyses (DCAs) to evaluate the performance of the nomogram. Statistical tests were performed with two-tailed p values, and P value < 0.05 was deemed statistically significant.
Results
Clinicopathological Characteristics
The mean patient age was 49.61 years (range: 12–84), and 133/702 (18.9%) were FTN and 569/702 (88.1%) were non-FTN. Table 2 lists the clinicopathological characteristics and MRI features of thyroid nodules in the training and validation cohort. There were no differences in clinicopathological features between the training cohort and the validation cohort except for lesion number (P = 0.026). In addition, among the 133 FTN patients with a mean age of 50.33 years (age range: 19–84), there were 116/133 (87.2%) BFTNs and 17/133 (12.8%) MFTNs. The BFTN group included 69/133 (51.9%) adenomatous goiter and 47/133 (35.3%) FTA. The MFTN group included 12/133 (9%) FTC and 5/133 (3.8%) FT-UMP.
Independent Predictive Factors of FTN and MFTN
Table 3 shows factors associated with FTN in the training cohort and associated with MFTN on the basis of the FTN samples. The kappa values for interobserver agreement for all MRI features were from 0.725 to 1.000. The independent predictors of FTN were pseudocapsule (P < 0.001; OR = 7.33), fissure-filling enhancement (P = 0.041; OR = 3.23), hyperintense on T2WI with enhancement (P < 0.001; OR = 8.05), uniformity of contrast enhancement (P < 0.001; OR = 4.37), and hyperenhancement in early phase (P < 0.001; OR = 20.71). Tumor size (P = 0.003; OR = 13.45), restricted diffusion (P < 0.001; OR = 22.22), and cystic degeneration (P = 0.001; OR = 13.05) were independent predictors of MFTN in multivariate analysis. Figure 2 presents forest plots showing the multivariate analysis for FTN and MFTN.
Development and Validation of the Nomogram for Predicting FTN
Figure 3 shows the nomogram for predicting FTN and the corresponding areas under the curve (AUC) in the training and validation cohorts were 0.947 (95% CI: 0.919–0.976) and 0.927 (95% CI: 0.876–0.978), respectively (Fig. 4a and d). Figure 4b and e demonstrates the calibration plots of the nomogram for predicting FTN in the training and validation cohorts. The consistencies between the predicted and the actual probability of FTN are favorable. The nomogram for predicting FTN indicated a good fit with a P value of 0.698 in the training cohort and 0.088 in the validation cohort. The decision curves of the nomogram for predicting FTN in the training and validation cohorts (Fig. 4c and f) showed good clinical utility. Figures 5 and 6 illustrate representative MRI images of patients with FTN.
The nomogram for predicting the probability of FTN based on MRI features. When using the nomogram, draw a vertical line to the corresponding point on the axis for each variable. Then, a total score line displays the summed points for each variable. An individual probability of FTN is obtained by projecting the total score line onto the predicted probability bottom scale
Performance and clinical utility of the nomogram for the discrimination of FTN and non-FTN. a ROC curve in training cohort (AUC: 0.947, 88% sensitivity and 92.4% specificity at the optimal cutoff value of 0.249); b Calibration curves in training cohort (Hosmer-Lemeshow test, P = 0.698); c Decision curves for the training cohort; d ROC curve in validation cohort (AUC: 0.927, 81.8% sensitivity and 92.5% specificity at the optimal cutoff value of 0.245); e Calibration curves in validation cohort (Hosmer-Lemeshow test, P = 0.088); f Decision curves for the validation cohort. ROC, receiver operating characteristic; AUC, area under the curve; FTN, follicular thyroid neoplasm
A 39-year-old woman presented with a thyroid follicular adenoma larger than 4 cm in the left lobe. Axial T2-weighted image a shows a heterogeneous mass and the present of hyperintense on T2WI with enhancement (black arrow). Axial DWI b and ADC c images show a hyperintense mass without restricted diffusion. Axial contrast-enhanced MRI (early phase) d shows a hyperintense enhancing mass and irregular fissure that did not enhance (black arrow). Axial contrast-enhanced MRI (delay phase) e shows a relatively homogeneous enhanced lesion with pseudocapsule, and fissure that do not enhanced in the early phase enhanced gradually. Pathological section analysis (HE, ×1) f shows densely distributed small follicles and relatively loose follicular cells (long black arrow, HE, ×40)
A 43-year-old man presented with a thyroid follicular carcinoma larger than 4 cm in the right lobe. Axial T2-weighted image a shows a heterogeneous mass and the present of hyperintense on T2WI with enhancement (black arrow). Axial DWI b and ADC c images show an area with restricted diffusion within the lesion (high signal on DWI and low signal on ADC, black arrow). Axial contrast-enhanced MRI (early phase) d shows a hyperintense enhancing mass and irregular fissure that did not enhance (black arrow). Axial contrast-enhanced MRI (delay phase) e shows a relatively homogeneous enhanced lesion with pseudocapsule (black arrow); fissure that do not enhanced in the early phase enhanced gradually, and the area of cystic degeneration is seen within the lesion. Pathological section analysis (HE, ×1) f shows densely distributed small follicles and follicular cells (long black arrow, HE, ×40)
Construction and Performance of the RSS for Predicting MFTN
The RSS based on regression β coefficients from the preceding multivariate regression model was developed as follows: FTNs were given two points for tumor size, three points for restricted diffusion, and two points for cystic degeneration (Table 4). The risk score was calculated to stratify FTNs into high-risk group and low-risk group according to the optimal cutoff value determined by the ROC method. The ROC curve was measured by bootstrapping, and the bootstrap-corrected AUC was 0.902 (95% CI: 0.798–0.971) (Fig. 7). Table 5 presents diagnostic performance and ROC curves of the RSS and five different TIRADS for predicting MFTN in 118 FTNs. Sensitivity, specificity, accuracy, PPV, NPV, and AUC of the RSS at the optimal cutoff value of 3.5 were 73.3%, 95.1%, 92.4%, 68.8%, 96.1%, and 0.842, respectively. The diagnostic performance of the RSS exceeded the performance of several other ultrasonography-based TIRADS (AUC: 0.504–0.643). Compared with five TIRADS, the RSS showed a significant improvement for predicting MFTN based on NRI analysis (40.78 − 76.38%, P < 0.05).
Discussion
In our study, we identified pseudocapsule, fissure-filling enhancement, hyperintense on T2WI with enhancement, uniformity of contrast enhancement, and hyperenhancement in early phase as key independent variables for FTN. The developed nomogram for FTN prediction demonstrated good discrimination performance, calibration, and clinical utility. For MFTN, tumor size, cystic degeneration, and restricted diffusion emerged as independent variables, with substantial interobserver agreement observed for these variables. Utilizing these aforesaid independent variables, we constructed RSS to predict MFTN. Comparative analysis using ROC and NRI indicated that the RSS outperformed the five main TIRADS classification systems in predicting MFTN.
The nomogram for FTN prediction exhibited impressive diagnosis performance in both training and validation cohorts. Current ultrasound-based malignancy risk stratification systems for thyroid nodules have proven inadequate in accurately characterizing FTNs, often resulting in unnecessary biopsies [16]. FTN patients might benefit from this nomogram by reducing the need for invasive biopsy. In our study, the presence of pseudocapsule emerged as a significant independent risk factor for FTN prediction, and appearing in 61% of FTNs in the training cohort, a higher prevalence compared to non-FTNs. The pseudocapsule is characterized as a fibrous connective tissue ring formed by tumor compression, which manifests as a halo sign on ultrasonography. Li et al. [27] reported that about 74% FTN had a pseudocapsule. We also found fissure-filling enhancement to be a distinctive feature, present in only 1.9% of non-FTNs but in about 42.9% of FTNs. Histologically, FTNs exhibit loose follicular structures, fibrous stroma, and hemorrhage internally, with fewer vessels, contrasting with densely distributed follicular structures, cells, and abundant vessels in the surrounding area. This contrast explains the pathological mechanism behind fissure-filling enhancement. Additionally, hyperintense on T2WI with enhancement and hyperenhancement in early phase were crucial in predicting FTN. The liquid-rich internal areas of FTNs contribute to hyperintense on T2WI with enhancement. The characteristic of dense area inside FTNs is that most of the cells are obviously crowded and/or accompanied by microfollicular formation [28]. These crowded cells contain proliferative-positive cells and, along with neovascularity, lead to hyperenhancement in early phase.
Differentiating MFTN from BFTN poses a significantly clinical, radiological, and histological challenge. The findings of our research indicated that the detection of MFTNs using the five ultrasound TIRADS stratification systems yielded a limited number of true positive cases, leading to reduced sensitivity and PPV. Supporting our observations, Lin et al.’s research into the efficacy of ultrasound-based malignancy risk stratification systems for thyroid nodules, specifically within FTN patients, revealed similar limitations. The sensitivity among K-TIRADS, EU-TIRADS, ACR-TIRADS, and C-TIRADS ranged from 26.9 to 38.8%, with the PPV ranging from 32.7 to 60% [16]. The AUC of five TIRADS stratification systems to predict MFTN ranged from 0.515 to 0.643 in our present study, which was comparable to findings of Lin et al. [16] who reported an AUC of 0.573 to 0.611. These results emphasized a broader challenge within the field in effectively diagnosing MFTNs through current TIRADS systems, thus highlighting the need for the urgent development of a malignant risk prediction model specially designed for FTNs. To address this, researchers have explored various preoperative methods to preoperatively distinguish FTC from FTA, such as serum-based analysis [29], radiomics and machine learning based on ultrasonography or CT images [5, 30, 31], and employing specific ultrasonography features [7, 27, 32, 33]. Nonetheless, the effectiveness of ultrasonography is constrained by physician experience, and its diagnostic performance is marked by notable interobserver variability [34]. In our study, we developed an RSS using multiparametric MRI features to differentiate MFTN from BFTN. The RSS exhibited a strong predictive capacity for MFTN, achieving an AUC value of 0.902.
Tumor size emerged as a crucial predictor for MFTN in our study. We categorized tumor size as a binary variable, setting the threshold at 4 cm. Aligning with Mu et al. [35], our results affirm tumor size as an independent predictor for MFTN corroborating previous research [36,37,38,39]. Additionally, we identified cystic degeneration as another independent predictor for MFTN. While cystic degeneration acts as a protective factor against malignancy in non-FTNs [40, 41], it poses a risk for malignancy in FTNs. Our findings are consistent with Ou et al. [33], whose results associated cystic degeneration with FTC. Contrasting results in other studies, particularly those focusing on ultrasonography features, might stem from varying definitions of cystic degeneration or differences in interobserver variability.
In oncology, diffusion-weighted imaging (DWI) is increasingly utilized for diagnosis, monitoring, and predicting outcome of malignancies [42]. DWI enables quantification of water diffusivity through the apparent diffusion coefficient (ADC), which is inversely correlated with cell density in tumor tissues [43]. ADC has become a valuable tool for distinguishing benign from malignant thyroid nodules [44]. However, despite the merits of the ADC, clinical practice seldom reports quantitative ADC values, and achieving consistent ADC measurements across different MRI scanners remains a challenge. We defined restricted diffusion as enhanced lesion areas appearing hyperintense on DWI and hypointense on ADC. Compared to quantitative ADC values, assessing restricted diffusion offers a practical and straightforward approach. In our study, restricted diffusion stood out as the most significant independent predictor. Pathologically, MFTN comprises of densely packed follicular cells, which limit the movement of water molecules.
Our study had several limitations. Firstly, as a single-center retrospective investigation, our results might be influenced by selection bias. Secondly, the small sample size of MFTN and the lack of analysis stratified by MFTN subtypes limit the conclusiveness of our findings; a larger MFTN sample is essential to validate the accuracy of the RSS for both benign and malignant FTN. Thirdly, while the MRI-based imaging features we identified are clinically practical, their subjectivity nature could have impacted our results. Lastly, reviewing static ultrasound images and reports may introduce bias, differing from real-life clinical practice.
Conclusion
In conclusion, our nomogram, integrating MRI-based imaging features, excelled in accurately predicting FTN preoperatively, offering a potential reduction in invasive biopsies. Additionally, the RSS developed for MFTN prediction could significantly aid clinicians in refining therapeutic decision-making.
Data Availability
The data sets generated and/or analyzed in the current study were not made public because patients’ personal information was included. Available from the corresponding author upon reasonable request.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- US:
-
Ultrasonography
- FTN:
-
Follicular thyroid neoplasm
- non-FTN:
-
Non-follicular thyroid neoplasm
- MFTN:
-
Malignant follicular thyroid neoplasm
- BFTN:
-
Benign follicular thyroid neoplasm
- FTA:
-
Follicular thyroid adenoma
- FTC:
-
Follicular thyroid carcinoma
- PTC:
-
Papillary thyroid carcinoma
- FNA:
-
Fine-needle aspiration
- FT-UMP:
-
Follicular tumors with uncertain malignant potential
- TIRADS:
-
Thyroid Imaging Reporting and Data System
- ACR-TIRADS:
-
American College of Radiology Thyroid Imaging Reporting and Data System
- K-TIRADS:
-
Korean Thyroid Imaging Reporting and Data System
- EU-TIRADS:
-
European Thyroid Imaging Reporting and Data System
- C-TIRADS:
-
Chinese Thyroid Imaging Reporting and Data System
- ATA:
-
American Thyroid Association
- T1WI:
-
T1 weighted imaging
- T2WI:
-
T2 weighted imaging
- CI:
-
Confidence intervals
- SD:
-
Standard deviation
- ROC:
-
Receiver operating characteristic
- DCA:
-
Decision curve analysis
- DWI:
-
Diffusion-weighted imaging
- ADC:
-
Apparent diffusion coefficient
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- NRI:
-
Net reclassification index
References
Angell TE: RAS-positive thyroid nodules. Current opinion in endocrinology, diabetes, and obesity 24:372–376, 2017
Patel SG, et al.: Preoperative detection of RAS mutation may guide extent of thyroidectomy. Surgery 161:168–175, 2017
Ito Y, et al.: Clinical outcomes of follicular tumor of uncertain malignant potential of the thyroid: real-world data. Endocr J 69:757–761, 2022
Machens A, Lorenz K, Weber F, Dralle H: Risk Patterns of Distant Metastases in Follicular, Papillary and Medullary Thyroid Cancer. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 54:7–11, 2022
Shin I, et al.: Application of machine learning to ultrasound images to differentiate follicular neoplasms of the thyroid gland. Ultrasonography 39:257–265, 2020
Kuo TC, Wu MH, Chen KY, Hsieh MS, Chen A, Chen CN: Ultrasonographic features for differentiating follicular thyroid carcinoma and follicular adenoma. Asian journal of surgery 43:339–346, 2020
Wu Q, Qu Y, Li Y, Liu Y, Shen J, Wang Y: Logistic regression analysis of contrast-enhanced ultrasound and conventional ultrasound of follicular thyroid carcinoma and follicular adenoma. Gland surgery 10:2890–2900, 2021
Huang L, et al.: Molecular Pathological Diagnosis of Thyroid Tumors Using Spatially Resolved Metabolomics. Molecules 27, 2022
Savala R, Dey P, Gupta N: Artificial neural network model to distinguish follicular adenoma from follicular carcinoma on fine needle aspiration of thyroid. Diagn Cytopathol 46:244–249, 2018
Tessler FN, et al.: ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. Journal of the American College of Radiology: JACR 14:587–595, 2017
Shin JH, et al.: Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean journal of radiology 17:370–395, 2016
Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L: European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. European thyroid journal 6:225–237, 2017
Kwak JY, et al.: Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 260:892–899, 2011
Zhou J, et al.: 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine 70:256–279, 2020
Kwak JY, et al.: Image reporting and characterization system for ultrasound features of thyroid nodules: multicentric Korean retrospective study. Korean journal of radiology 14:110–117, 2013
Lin Y, et al.: Performance of current ultrasound-based malignancy risk stratification systems for thyroid nodules in patients with follicular neoplasms. European radiology 32:3617–3630, 2022
Li HJ, Yang YP, Liang X, Zhang Z, Xu XH: Comparison of the diagnostic performance of three ultrasound thyroid nodule risk stratification systems for follicular thyroid neoplasm: K-TIRADS, ACR -TIRADS and C-TIRADS. Clinical hemorheology and microcirculation, 2023
Haugen BR, et al.: 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid: official journal of the American Thyroid Association 26:1–133, 2016
Alizad A, et al.: In vivo thyroid vibro-acoustography: a pilot study. BMC medical imaging 13:12, 2013
Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS: The Diagnosis and Management of Thyroid Nodules: A Review. Jama 319:914–924, 2018
Bi Q, et al.: The Diagnostic Value of MRI for Preoperative Staging in Patients with Endometrial Cancer: A Meta-Analysis. Acad Radiol 27:960–968, 2020
Cho SJ, Suh CH, Baek JH, Chung SR, Choi YJ, Lee JH: Diagnostic performance of MRI to detect metastatic cervical lymph nodes in patients with thyroid cancer: a systematic review and meta-analysis. Clinical radiology 75:562.e561-562.e510, 2020
Hu W, et al.: MRI-based radiomics analysis to predict preoperative lymph node metastasis in papillary thyroid carcinoma. Gland surgery 9:1214–1226, 2020
Qin H, et al.: Magnetic resonance imaging (MRI) radiomics of papillary thyroid cancer (PTC): a comparison of predictive performance of multiple classifiers modeling to identify cervical lymph node metastases before surgery. La Radiologia medica 126:1312–1327, 2021
Wang H, et al.: Machine learning-based multiparametric MRI radiomics for predicting the aggressiveness of papillary thyroid carcinoma. Eur J Radiol 122:108755, 2020
Wei R, et al.: Radiomics based on multiparametric MRI for extrathyroidal extension feature prediction in papillary thyroid cancer. BMC medical imaging 21:20, 2021
Li W, et al.: The Value of Sonography in Distinguishing Follicular Thyroid Carcinoma from Adenoma. Cancer Manag Res 13:3991–4002, 2021
Cibas ES, Ali SZ: The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 27:1341–1346, 2017
Chen Z, et al.: The utility of serum anti-thyroglobulin antibody and thyroglobulin in the preoperative differential diagnosis of thyroid follicular neoplasms. Endocrine 76:369–376, 2022
Tang P, Ren C, Shen L, Zhou Z: Development and Validation of a Diagnostic Nomogram for the Preoperative Differentiation Between Follicular Thyroid Carcinoma and Follicular Thyroid Adenomas. J Comput Assist Tomogr 45:128–134, 2021
Yu B, et al.: Differentiate Thyroid Follicular Adenoma from Carcinoma with Combined Ultrasound Radiomics Features and Clinical Ultrasound Features. Journal of digital imaging 35:1362–1372, 2022
Huang Q, et al.: Development and Validation of an Ultrasonic Diagnostic Model for Differentiating Follicular Thyroid Carcinoma from Follicular Adenoma. International journal of general medicine 14:5069–5078, 2021
Ou D, et al.: Ultrasonic identification and regression analysis of 294 thyroid follicular tumors. Journal of cancer research and therapeutics 16:1056–1062, 2020
Yoo YJ, Ha EJ, Cho YJ, Kim HL, Han M, Kang SY: Computer-Aided Diagnosis of Thyroid Nodules via Ultrasonography: Initial Clinical Experience. Korean journal of radiology 19:665–672, 2018
Mu N, et al.: High Ki-67 index in fine needle aspiration cytology of follicular thyroid tumors is associated with increased risk of carcinoma. Endocrine 61:293–302, 2018
Boonrod A, et al.: Thyroid Nodule Size as a Predictor of Malignancy in Follicular and Hurthle Neoplasms. Asian Pacific journal of cancer prevention: APJCP 22:2597–2602, 2021
Borowczyk M, et al.: Sonographic Features Differentiating Follicular Thyroid Cancer from Follicular Adenoma-A Meta-Analysis. Cancers 13, 2021
Cordes M, Nagel H, Horstrup K, Sasiadek M, Kuwert T: Ultrasound characteristics of thyroid nodules diagnosed as follicular neoplasms by fine-needle aspiration cytology. A prospective study with histological correlation. Nuklearmedizin Nuclear medicine 55:93–98, 2016
Sillery JC, Reading CC, Charboneau JW, Henrichsen TL, Hay ID, Mandrekar JN: Thyroid follicular carcinoma: sonographic features of 50 cases. AJR American journal of roentgenology 194:44–54, 2010
Na DG, Kim JH, Kim DS, Kim SJ: Thyroid nodules with minimal cystic changes have a low risk of malignancy. Ultrasonography 35:153–158, 2016
Wang H, Wei R, Liu W, Chen Y, Song B: Diagnostic efficacy of multiple MRI parameters in differentiating benign vs. malignant thyroid nodules. BMC medical imaging 18:50, 2018
Liu Y, et al.: Comparative Study of Monoexponential, Intravoxel Incoherent Motion, Kurtosis, and IVIM-Kurtosis Models for the Diagnosis and Aggressiveness Assessment of Prostate Cancer. Frontiers in oncology 10:1763, 2020
Chan SC, Yeh CH, Chang JT, Chang KP, Wang JH, Ng SH: Combing MRI Perfusion and (18)F-FDG PET/CT Metabolic Biomarkers Helps Predict Survival in Advanced Nasopharyngeal Carcinoma: A Prospective Multimodal Imaging Study. Cancers 13, 2021
Chen L, et al.: Diffusion-weighted MRI in differentiating malignant from benign thyroid nodules: a meta-analysis. BMJ open 6:e008413, 2016
Acknowledgements
The authors thank AiMi Academic Services (www.aimieditor.com) for the English language editing and review services.
Funding
This work was supported by the Nature Science Foundation of Shanghai (grant number: 19ZR1446200), Science and Technology Commission of Minhang District, Shanghai (grant number: 2020MHZ034) and Shanghai Municipal Health Commission (grant number: 202140325).
Author information
Authors and Affiliations
Contributions
All authors and co-authors have significantly contributed. Bin Song: data analysis and writing and review of manuscript. Tingting Zheng: data collection and writing and editing of manuscript. Hao Wang: contribution of data and review of manuscript. Lang Tang: contribution to ultrasound data. Xiaoli Xie: contribution to pathologic data. Qingyin Fu: contribution to ultrasound data. Weiyan Liu: contribution to data and thyroid surgery. Pu-Yeh Wu: contribution to review and editing of manuscript. Wenjuan Hu: contribution to data and review and editing of manuscript. Mengsu Zeng: study design, review of manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The institutional review board of Minhang hospital, Fudan University approved this study.
Consent to Participate
Informed consent was exempted due to the retrospective nature.
Consent to Publish
Informed consent was waived because it was a retrospective study and the images we used did not include personal information.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, B., Zheng, T., Wang, H. et al. Prediction of Follicular Thyroid Neoplasm and Malignancy of Follicular Thyroid Neoplasm Using Multiparametric MRI. J Digit Imaging. Inform. med. (2024). https://doi.org/10.1007/s10278-024-01102-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10278-024-01102-0