Abstract
This study aimed to investigate the effects of intravenous injection of iodine contrast agent on the tracheal diameter and lung volume. In this retrospective study, a total of 221 patients (71.1 ± 12.4 years, 174 males) who underwent vascular dynamic CT examination including chest were included. Unenhanced, arterial phase, and delayed-phase images were scanned. The tracheal luminal diameters at the level of the thoracic inlet and both lung volumes were evaluated by a radiologist using a commercial software, which allows automatic airway and lung segmentation. The tracheal diameter and both lung volumes were compared between the unenhanced vs. arterial and delayed phase using a paired t-test. The Bonferroni correction was performed for multiple group comparisons. The tracheal diameter in the arterial phase (18.6 ± 2.4 mm) was statistically significantly smaller than those in the unenhanced CT (19.1 ± 2.5 mm) (p < 0.001). No statistically significant difference was found in the tracheal diameter between the delayed phase (19.0 ± 2.4 mm) and unenhanced CT (p = 0.077). Both lung volumes in the arterial phase were 4131 ± 1051 mL which was significantly smaller than those in the unenhanced CT (4332 ± 1076 mL) (p < 0.001). No statistically significant difference was found in both lung volumes between the delayed phase (4284 ± 1054 mL) and unenhanced CT (p = 0.068). In conclusion, intravenous infusion of iodine contrast agent transiently decreased the tracheal diameter and both lung volumes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the radiology department, contrast media is widely used. For CT and MRI examinations, iodine- and gadolinium-based contrast media, respectively, are used. However, the use of contrast media is associated with some adverse reactions. For both the iodine [1] and gadolinium-based contrast media [2], the most common major adverse reaction is hypersensitivity reaction. For patients with renal function impairment, radiologists need to exercise caution regarding contrast-induced nephropathy [3] and nephrogenic systemic fibrosis [4] from the iodine and gadolinium-based contrast media, respectively. For pregnant patients, gadolinium-based contrast media is associated with some adverse reactions including stillbirth or neonatal death [5]. Recently, two other new phenomena have been discovered: central nervous system cumulation of gadolinium for gadolinium-based contrast agent [6] and acute transient dyspnea after intravenous injection of gadoxetate disodium and gadobenate dimeglumine [7]. Additionally, we coincidentally found other new phenomenon associated with intravenous infusion of iodine contrast media, that is, a transient decrease in the tracheal diameter and lung volume in the arterial phase.
Airway diameter is associated with some diseases, especially asthma. In asthma, loss of homeostatic control of the airway smooth muscle causes hypercontractility with an increased risk for bronchospasm. A bronchodilator is used to manage this disease [8]. Additionally, a risk factor for adverse reactions in contrast agent use is poorly controlled bronchial asthma [9].
Owing to the deep-learning advancements in the field of radiology [10,11,12], an accurate evaluation of the lung volume became possible [13,14,15]. In the evaluation of several conditions, such as interstitial lung disease [16, 17], chronic obstructive pulmonary disease [18, 19], coronavirus pneumonia [20], size matching prior to lung transplantation [21], and detection of bronchiolitis obliterans in patients after lung transplantation [22], the utility of CT lung volumetry was reported in some studies.
Thus, fundamental data regarding the relationship between the infusion of iodine contrast agent vs. tracheal diameter and lung volume would be necessary. In the evaluation of this relationship, chest CT scans of both the unenhanced and contrast-enhanced CT performed within a single CT examination are ideal. In our hospital, vascular dynamic CT including chest, in which the unenhanced, arterial, and delayed phase are scanned in a single examination, satisfies this criterion. This study aims to investigate the effects of intravenous infusion of contrast material on the tracheal diameter and lung volume using chest dynamic CT images including unenhanced, arterial, and delayed phase performed within a single CT examination.
Materials and Methods
Our Institutional Review Board approved this retrospective study, which waived the requirement for obtaining written informed consent from patients.
Patients
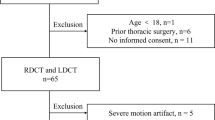
Patients who underwent vascular dynamic CT examination including the chest (unenhanced, arterial phase, and delayed phase) from January 2021 to February 2023 were included in this study. The following patients were excluded: (a) error in automatic segmentation of the lung or bronchus (n = 3) and (b) lost data regarding contrast material (n = 1). The patient inclusion and exclusion processes are illustrated in Fig. 1.
CT Imaging
Patients underwent CT examination using CT scanners from two vendors (Canon Medical Systems [Tochigi, Japan] and GE Healthcare [WI, US]). Clinical indications for CT examination were the following: evaluations of abdominal aortic aneurysm (n = 109), peripheral arterial disease (n = 46), aneurysm of abdominal branches (n = 29), aortic dissection (n = 14), thoracic aortic aneurysm (n = 10), and others (n = 13). The CT scanning parameters were as follows: tube voltage at 120 kVp and tube current with automatic tube current modulation with standard deviation/noise index set at 13.0 for Canon-CT and 11.36 for GE-CT. The reconstruction parameters were the following: field of view which is adjusted to body size, slice thickness/interval of 1–1.25 mm/0.8 mm, and the kernel of FC04 for Canon-CT and STANDARD for GE-CT. Patients were instructed to hold deep-inspiratory level during the scan for all phases. These parameters were the same across unenhanced, arterial, and delayed phase.
The contrast agent was injected via the right or left antecubital vein within 30 s. The timing of the arterial phase imaging was determined through real-time monitoring of contrast enhancement by placing region of interest on the descending aorta at the level of the diaphragm. Scan was started when the CT attenuation of the aorta reached 250 Hounsfield unit. The delayed phase was scanned at 90 s after administration of the contrast agent injection.
CT Image Evaluation
A radiologist with a 13-year diagnostic imaging experience performed the CT image evaluation using a commercial software (Synapse Vincent, Fujifilm [Tokyo, Japan]). This software, which was developed based on the deep-learning algorithm, allowed segmentation of each lobe of the lung as well as the entire lung fully automatically (Fig. 2a). The radiologist recorded the CT lung volume for each lobe including both lung volumes. Then, automatic segmentation of the airway was also performed. With this software, the inner lumen diameter of the trachea, which is averaged for the long axis and short axis, is displayed (Fig. 2b). The averaged tracheal diameter at the thoracic inlet was recorded.
Evaluation of the a lung volume and b tracheal diameter using a software. a The right upper lobe, right middle lobe, right lower lobe, left upper lobe, and left lower lobe were segmented and highlighted with yellow, blue, green, red, and pick, respectively. b Trachea at the thoracic inlet is annotated with pink arrows in the left CT image. Inner lumen and outer boundary are shown with green and red lines, respectively. Inner lumen diameter is also displayed as pink numerals
The arterial phase to the unenhanced and delayed phase to the unenhanced ratio for the tracheal diameter (TDAU and TDDU, respectively), both lung volumes (BLVAU and BLVDU, respectively), and each lobe volume were calculated.
Statistical Analysis
Statistical analysis was performed using R version 4.1.2 (https://www.r-project.org/). The tracheal diameter and both lung volumes were compared between the unenhanced CT vs. the arterial phase and unenhanced vs. delayed phase using the paired t-test. The Bonferroni correction was performed for these analyses because multiple groups were compared. Correlations between the patient’s age vs. the tracheal diameter and both lung volumes were assessed using the Pearson’s correlation coefficient. Associations between background factors vs. the tracheal diameter and both lung volumes were assessed using a Student’s t-test or analysis of variance. Statistical significance was set at a p value < 0.05.
Results
Patients
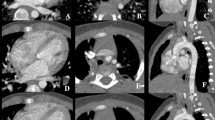
Patient background information is described in Table 1. A total of 221 patients (71.1 ± 12.4 years, 174 males) were included. Representative vascular dynamic CT images are shown in Fig. 3.
Representative CT image of a 61-year-old female patient who was administered with an iodine contrast agent (iopamidol, 370 mgI/mL) from the right antecubital vein. Both lung volumes in the a unenhanced, b arterial phase, and c delayed phase images were 2144, 1739, and 2434 mL, respectively. The tracheal diameters at the thoracic inlet in the unenhanced, arterial phase, and delayed phase were 13.6, 12.6, and 14.3 mm, respectively
Tracheal Diameter and Lung Volume Comparisons Between Each Phase
The detailed results of the tracheal diameter and lung volume are provided in Table 2. Bland–Altman plots are shown in Fig. 4. The tracheal diameter in the arterial phase was 18.6 ± 2.4 mm which was significantly smaller than those in the unenhanced CT (19.1 ± 2.5 mm) (p < 0.001). Even when the outliers with a difference > 3 mm as seen in Fig. 4a were excluded, the average tracheal diameters in unenhanced (19.1 mm) and arterial phase (18.6 mm) have not changed, and there was still a statistically significant difference between them (p < 0.001). There was no considerable misregistration by the software for these patients (Fig. 5). No statistically significant difference was found in the tracheal diameter between the delayed phase (19.0 ± 2.4 mm) and unenhanced CT (p = 0.077).
Bland–Altman plots for the a tracheal diameter between the arterial and unenhanced, b both lung volumes between the arterial and unenhanced, c tracheal diameter between the delayed phase and unenhanced, and d both lung volumes between the delayed phase and unenhanced. Dashed and solid lines indicate mean of difference and limits of agreement, respectively
Both lung volumes in the arterial phase were 4131 ± 1051 mL which was significantly smaller than those in the unenhanced CT (4332 ± 1076 mL) (p < 0.001). Conversely, no statistically significant difference was found in the volume of both lungs between the delayed phase (4284 ± 1054 mL) and unenhanced CT (p = 0.068).
Data regarding the TDAU, TDDU, BLVAU, and BLVDU values are described in Table 3. The tracheal diameter and both lung volumes in the arterial phase were decreased by 2.5% and 4.4%, respectively, compared with the unenhanced CT. In the arterial phase, a more than 10% decrease in the tracheal diameter was observed for 1.8% of patients. The lung volume was decreased in more than 10% in 18.1% of patients.
Significant positive correlations exist between TDAU vs. BLVAU (r = 0.533 [95% confidence interval, 0.432–0.622], p < 0.001) and TDDU vs. BLVDU (r = 0.464 [95% confidence interval, 0.353–0.561], p < 0.001).
Arterial to Unenhanced and Delayed to Unenhanced Ratios of Each Lobe Volume
The arterial phase to unenhanced ratio for the right upper lobe, right middle lobe, right lower lobe, left upper lobe, and left lower lobe was 0.971 ± 0.075, 0.985 ± 0.076, 0.941 ± 0.135, 0.966 ± 0.076, and 0.931 ± 0.137, respectively. The volume of the right lower lobe and left lower lobe, which is located near the diaphragm, was more largely affected by the intravenous infusion of contrast material than the upper and middle lobes.
The delayed phase to unenhanced ratio for the right upper lobe, right middle lobe, right lower lobe, left upper lobe, and left lower lobe was 0.995 ± 0.063, 1.010 ± 0.053, 0.992 ± 0.134, 0.994 ± 0.065, and 0.991 ± 0.135, respectively.
Factors Affecting the Arterial to Unenhanced Ratio for the Tracheal Diameter and Lung Volume
The detailed results for the association between each background factor vs. TDAU and BLVAU are described in Table 4. The right-side injection was found to be significantly associated with a lower BLVAU value (0.950 ± 0.091) compared with the left-side injection (0.988 ± 0.086) (p = 0.022). No significant factor was found to affect the TDAU. The patients’ age, sex, and type or iodine concentration of the contrast material did not have a significant impact on the TDAU or BLVAU values (p > 0.300).
Discussion
Allergic reaction and contrast-induced nephropathy are well-known adverse reactions associated with iodine contrast material. Additionally, we found and confirmed a new phenomenon: a transient decrease in the tracheal diameter and lung volume. The tracheal diameter and both lung volumes were decreased by 2.5% and 4.4%, respectively, in the arterial phase, and a more than 10%/20% decrease of tracheal diameter and both lung volumes was observed in 1.8%/0.5% and 18.1%/3.6% of patients, respectively. These phenomena were transient, and no significant effect was observed in the delayed phase.
There have been studies which reported factors affecting the diameter or airway volume. Yamada et al. reported that the ratio of inspiratory to expiratory airway volume in supine position is larger than the standing position in patients with chronic obstructive pulmonary disease (p < 0.001) [19]. In asthmatics, for whom the bronchoalveolar lavage fluid count tend to be high, the eosinophil count in the bronchoalveolar lavage fluid was negatively associated with the airway diameter (r = − 0.7, p < 0.05) [23]. Additionally, while obesity is known to be associated with a higher incidence and prevalence of asthma [24], abdominal visceral fat area was negatively associated with airway diameter in asthmatics (r = − 0.35, p = 0.01) [25]. Furthermore, the barometric pressure has an impact on the increased risk for hospital visit for asthma [26]. Our study is unique in that the association between contrast agent injection, which is known to be a risk factor for patients with poorly controlled asthma [9], and decrease in tracheal diameter was demonstrated. Whether this phenomenon can be a trigger for hypersensitivity reaction in patients with or without asthma requires further investigation.
Chest CT examination is sometimes performed at levels other than end-inspiratory level. One representative case is CT pulmonary angiography, which is sometimes scanned at mid-inspiratory level to reduce the risk of transient interruption of contrast [27]. However, chest CT image scanned at mid-inspiratory level is known to increase a risk of nondiagnostic lung images [27]. From our study, it was found that more than 10% or 20% decrease of both lung volumes was observed in 18.1% and 3.6% of patients, respectively. In daily clinical practice, it would be better to avoid scanning chest at the arterial phase when aimed to evaluate the lung at the end-inspiratory level.
In interstitial lung diseases, it has been reported that both lung volumes were 3345 mL, and the relative annual bilateral lung volume loss was reportedly 2.07% and 17.44% in patients without and with major adverse event at a 3-year follow-up, respectively [17]. In our study, it was found that both lung volumes decreased by an average of 4.4% for the arterial phase compared with the unenhanced CT, and more than 10% and 20% decrease was observed in 18.1% and 3.6% of patients, respectively. The effect of a transient decrease in both lung volumes in the arterial phase would not be negligible in the lung volume assessment of interstitial lung disease.
The ratio of the expiratory volume to inspiratory volume in the lower lobes has been reportedly smaller than those in the upper and middle lobes (41.1–41.7% vs. 51.9–65.4% [28] or 57.4–57.8% vs. 67.5–74.1% [19]). This would be attributed to the fact that the lower lobes are located near the diaphragm which play an important role in respiration. In the current study, the volume of the lower lobes was more largely decreased (93.1–94.1%) in the arterial phase compared with the upper or middle lobes (96.6–98.5%). This fact indicates that the lobe volume decrease in the arterial phase was caused by the movement of the diaphragm. In addition, not only the lung volume but also the tracheal diameter was decreased in the arterial phase, and significant correlation between TDAU and BLVAU was observed. Furthermore, we excluded patients in whom errors in segmentation of the lung or bronchus were seen. These facts suggest that the change in the arterial phase was caused by the respiration not merely by the segmentation performance variation caused by the CT attenuation of the pulmonary vessels.
The right-side injection was significantly associated with a lower BLVAU value (0.950) compared with the left-side injection (0.988) (p = 0.022). This may be associated with the fact that the length between the antecubital vein and the right side of the heart is shorter for the right antecubital vein. A transient dilatation of right side of the heart or transient chemical reaction caused by the contrast agent molecule may be possible reasons for this phenomenon; however, a detailed mechanism remains unknown.
This study has some limitations. First, the relationship between the hypersensitivity reaction secondary to the contrast agent and the tracheal diameter was not assessed in this study. Future study regarding this topic is warranted. Second, because a CT scan was performed during end-inspiration level, whether change could be observed even in resting breathing state or patients just experienced difficulty in deep inspiration remains unclear. Third, because patients included in this study did not necessarily have lung diseases, depiction of diseases was not assessed. Finally, all patients included in this study underwent vascular dynamic CT examinations. Future studies including patients with other conditions are warranted.
In conclusion, the tracheal diameter and both lung volumes were transiently decreased in the arterial phase of vascular dynamic CT examination compared to unenhanced CT. To reveal the onset and duration of this phenomenon, future studies including CT examinations other than vascular dynamic CT is needed. In addition, the mechanism of this phenomenon is needed to be investigated in future studies.
Abbreviations
- BLVAU :
-
Arterial phase to unenhanced ratio for both lung volumes
- BLVDU :
-
Delayed phase to unenhanced ratio for both lung volumes
- TDAU :
-
Arterial phase to unenhanced ratio for the tracheal diameter
- TDDU :
-
Delayed phase to unenhanced ratio for the tracheal diameter
References
Umakoshi H, Nihashi T, Takada A et al. (2022) Iodinated Contrast Media Substitution to Prevent Recurrent Hypersensitivity Reactions: A Systematic Review and Meta-Analysis. Radiology 305(2):341-349. https://doi.org/10.1148/radiol.220370.
Behzadi AH, Zhao Y, Farooq Z, Prince MR (2018) Immediate Allergic Reactions to Gadolinium-based Contrast Agents: A Systematic Review and Meta-Analysis. Radiology 286(2):471-482. https://doi.org/10.1148/radiol.2017162740.
McDonald JS, McDonald RJ, Comin J et al. (2013) Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology 267(1):119-128. https://doi.org/10.1148/radiol.12121460.
Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE (2011) Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol 65(6):1095–1106 e1097. https://doi.org/10.1016/j.jaad.2010.08.041.
Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL (2016) Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA 316(9):952-961. https://doi.org/10.1001/jama.2016.12126.
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270(3):834-841. https://doi.org/10.1148/radiol.13131669.
Davenport MS, Viglianti BL, Al-Hawary MM et al. (2013) Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 266(2):452-461. https://doi.org/10.1148/radiol.12120826.
Porsbjerg C, Melen E, Lehtimaki L, Shaw D (2023) Asthma. Lancet 401(10379):858-873. https://doi.org/10.1016/S0140-6736(22)02125-0.
Rosado Ingelmo A, Dona Diaz I, Cabanas Moreno R et al. (2016) Clinical Practice Guidelines for Diagnosis and Management of Hypersensitivity Reactions to Contrast Media. J Investig Allergol Clin Immunol 26(3):144–155; quiz 142 p following 155. https://doi.org/10.18176/jiaci.0058.
Yasaka K, Akai H, Kunimatsu A, Kiryu S, Abe O (2018) Deep learning with convolutional neural network in radiology. Jpn J Radiol 36(4):257-272. https://doi.org/10.1007/s11604-018-0726-3.
Chartrand G, Cheng PM, Vorontsov E et al. (2017) Deep Learning: A Primer for Radiologists. Radiographics 37(7):2113-2131. https://doi.org/10.1148/rg.2017170077.
Yasaka K, Abe O (2018) Deep learning and artificial intelligence in radiology: Current applications and future directions. PLoS Med 15(11):e1002707. https://doi.org/10.1371/journal.pmed.1002707.
Park J, Yun J, Kim N et al. (2020) Fully Automated Lung Lobe Segmentation in Volumetric Chest CT with 3D U-Net: Validation with Intra- and Extra-Datasets. J Digit Imaging 33(1):221-230. https://doi.org/10.1007/s10278-019-00223-1.
Yun J, Park J, Yu D et al. (2019) Improvement of fully automated airway segmentation on volumetric computed tomographic images using a 2.5 dimensional convolutional neural net. Med Image Anal 51:13-20. https://doi.org/10.1016/j.media.2018.10.006.
Dong X, Lei Y, Wang T et al. (2019) Automatic multiorgan segmentation in thorax CT images using U-net-GAN. Med Phys 46(5):2157-2168. https://doi.org/10.1002/mp.13458.
Robbie H, Wells AU, Jacob J et al. (2019) Visual and Automated CT Measurements of Lung Volume Loss in Idiopathic Pulmonary Fibrosis. AJR Am J Roentgenol 213(2):318-324. https://doi.org/10.2214/AJR.18.20884.
Si-Mohamed SA, Nasser M, Colevray M et al. (2022) Automatic quantitative computed tomography measurement of longitudinal lung volume loss in interstitial lung diseases. Eur Radiol 32(6):4292-4303. https://doi.org/10.1007/s00330-021-08482-9.
Arjomandi M, Zeng S, Barjaktarevic I et al. (2019) Radiographic lung volumes predict progression to COPD in smokers with preserved spirometry in SPIROMICS. Eur Respir J 54(4). https://doi.org/10.1183/13993003.02214-2018.
Yamada Y, Chubachi S, Yamada M et al. (2022) Comparison of Lung, Lobe, and Airway Volumes between Supine and Upright Computed Tomography and Their Correlation with Pulmonary Function Test in Patients with Chronic Obstructive Pulmonary Disease. Respiration 101(12):1110-1120. https://doi.org/10.1159/000527067.
Ippolito D, Ragusi M, Gandola D et al. (2021) Computed tomography semi-automated lung volume quantification in SARS-CoV-2-related pneumonia. Eur Radiol 31(5):2726-2736. https://doi.org/10.1007/s00330-020-07271-0.
Shepherd HM, Farahnak K, Harrison MS et al. (2023) Utilizing computed tomography volumetry for size matching prior to lung transplantation: a case series. J Thorac Dis 15(4):2233-2239. https://doi.org/10.21037/jtd-22-1203.
Dettmer S, Suhling H, Klingenberg I et al. (2018) Lobe-wise assessment of lung volume and density distribution in lung transplant patients and value for early detection of bronchiolitis obliterans syndrome. Eur J Radiol 106:137-144. https://doi.org/10.1016/j.ejrad.2018.07.016.
Gorska K, Krenke R, Kosciuch J et al. (2009) Relationship between airway inflammation and remodeling in patients with asthma and chronic obstructive pulmonary disease. Eur J Med Res 14 Suppl 4(Suppl 4):90–96. https://doi.org/10.1186/2047-783x-14-s4-90.
Dixon AE, Holguin F, Sood A et al. (2010) An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc 7(5):325-335. https://doi.org/10.1513/pats.200903-013ST.
Yang MS, Choi S, Choi Y, Jin KN (2018) Association Between Airway Parameters and Abdominal Fat Measured via Computed Tomography in Asthmatic Patients. Allergy Asthma Immunol Res 10(5):503-515. https://doi.org/10.4168/aair.2018.10.5.503.
Zhang H, Liu S, Chen Z, Zu B, Zhao Y (2020) Effects of variations in meteorological factors on daily hospital visits for asthma: A time-series study. Environ Res 182:109115. https://doi.org/10.1016/j.envres.2020.109115.
Kay FU, Macedo AC, Chate RC et al. (2014) Reduction of poor contrast enhancement of the pulmonary artery in computed tomography angiography using an alternative respiratory maneuver. J Thorac Imaging 29(2):107-112. https://doi.org/10.1097/RTI.0000000000000050.
Wu F, Chen L, Huang J et al. (2021) Total Lung and Lobar Quantitative Assessment Based on Paired Inspiratory-Expiratory Chest CT in Healthy Adults: Correlation with Pulmonary Ventilatory Function. Diagnostics (Basel) 11(10). https://doi.org/10.3390/diagnostics11101791.
Funding
Open Access funding provided by The University of Tokyo. This work was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This retrospective study was approved by our research ethics committee.
Informed Consent
The requirement for informed consent was waived due to the retrospective nature of this study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yasaka, K., Saigusa, H. & Abe, O. Effects of Intravenous Infusion of Iodine Contrast Media on the Tracheal Diameter and Lung Volume Measured with Deep Learning-Based Algorithm. J Digit Imaging. Inform. med. (2024). https://doi.org/10.1007/s10278-024-01071-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10278-024-01071-4