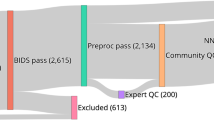
Abstract
This paper presents a fully automated pipeline using a sparse convolutional autoencoder for quality control (QC) of affine registrations in large-scale T1-weighted (T1w) and T2-weighted (T2w) magnetic resonance imaging (MRI) studies. Here, a customized 3D convolutional encoder-decoder (autoencoder) framework is proposed and the network is trained in a fully unsupervised manner. For cross-validating the proposed model, we used 1000 correctly aligned MRI images of the human connectome project young adult (HCP-YA) dataset. We proposed that the quality of the registration is proportional to the reconstruction error of the autoencoder. Further, to make this method applicable to unseen datasets, we have proposed dataset-specific optimal threshold calculation (using the reconstruction error) from ROC analysis that requires a subset of the correctly aligned and artificially generated misalignments specific to that dataset. The calculated optimum threshold is used for testing the quality of remaining affine registrations from the corresponding datasets. The proposed framework was tested on four unseen datasets from autism brain imaging data exchange (ABIDE I, 215 subjects), information eXtraction from images (IXI, 577 subjects), Open Access Series of Imaging Studies (OASIS4, 646 subjects), and “Food and Brain” study (77 subjects). The framework has achieved excellent performance for T1w and T2w affine registrations with an accuracy of 100% for HCP-YA. Further, we evaluated the generality of the model on four unseen datasets and obtained accuracies of 81.81% for ABIDE I (only T1w), 93.45% (T1w) and 81.75% (T2w) for OASIS4, and 92.59% for “Food and Brain” study (only T1w) and in the range 88–97% for IXI (for both T1w and T2w and stratified concerning scanner vendor and magnetic field strengths). Moreover, the real failures from “Food and Brain” and OASIS4 datasets were detected with sensitivities of 100% and 80% for T1w and T2w, respectively. In addition, AUCs of > 0.88 in all scenarios were obtained during threshold calculation on the four test sets.






Similar content being viewed by others
Data Availability
The datasets analyzed during the current study are available in the HCP-YA repository, https://www.humanconnectome.org/study/hcp-young-adult/overview; ABIDE I, https://fcon_1000.projects.nitrc.org/indi/abide/abide_I.html; OASIS4, https://www.oasis-brains.org/#data; Food and Brain; https://openneuro.org/datasets/ds004697/versions/1.0.1.
References
Eliot L, Ahmed A, Khan H, Patel J (2021) Dump the “dimorphism”: Comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neurosci Biobehav Rev 125:667–697. https://doi.org/10.1016/j.neubiorev.2021.02.026
Pomponio R, Erus G, Habes M, et al (2020) Harmonization of large MRI datasets for the analysis of brain imaging patterns throughout the lifespan. Neuroimage 208:116450. https://doi.org/10.1016/j.neuroimage.2019.116450
Liew S-L, Zavaliangos-Petropulu A, Jahanshad N, et al (2022) The ENIGMA Stroke Recovery Working Group: Big data neuroimaging to study brain–behavior relationships after stroke. Hum Brain Mapp 43:129–148. https://doi.org/10.1002/hbm.25015
Alfaro-Almagro F, Jenkinson M, Bangerter NK, et al (2018) Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage 166:400–424. https://doi.org/10.1016/j.neuroimage.2017.10.034
Poldrack RA, Gorgolewski KJ (2014) Making big data open: data sharing in neuroimaging. Nat Neurosci 17:1510–1517. https://doi.org/10.1038/nn.3818
Chen S, He Z, Han X, et al (2019) How Big Data and High-performance Computing Drive Brain Science. Genomics Proteomics Bioinformatics 17:381–392. https://doi.org/10.1016/j.gpb.2019.09.003
Di Martino A, Yan C-G, Li Q, et al (2014) The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry 19:659–667. https://doi.org/10.1038/mp.2013.78
Marcus DS, Wang TH, Parker J, et al (2007) Open Access Series of Imaging Studies (OASIS): Cross-sectional MRI Data in Young, Middle Aged, Nondemented, and Demented Older Adults. J Cogn Neurosci 19:1498–1507. https://doi.org/10.1162/jocn.2007.19.9.1498
Van Essen DC, Smith SM, Barch DM, et al (2013) The WU-Minn Human Connectome Project: An overview. Neuroimage 80:62–79. https://doi.org/10.1016/j.neuroimage.2013.05.041
Weiner MW, Veitch DP, Aisen PS, et al (2012) The Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement 8. https://doi.org/10.1016/J.JALZ.2011.09.172
Food and Brain Study - OpenNeuro. https://openneuro.org/datasets/ds004697/versions/1.0.1. Accessed 2 Oct 2023
Helms G, Kallenberg K, Dechent P (2006) Contrast-driven approach to intracranial segmentation using a combination of T2- and T1-weighted 3D MRI data sets. Journal of Magnetic Resonance Imaging 24:790–795. https://doi.org/10.1002/JMRI.20692
Denis De Senneville B, Manjón J V., Coupé P (2020) RegQCNET: Deep quality control for image-to-template brain MRI affine registration. Phys Med Biol 65. https://doi.org/10.1088/1361-6560/ABB6BE
Tummala S, Thadikemalla VSG, Kreilkamp BAK, et al (2021) Fully automated quality control of rigid and affine registrations of T1w and T2w MRI in big data using machine learning. Comput Biol Med 139:104997. https://doi.org/10.1016/j.compbiomed.2021.104997
Bottani S, Burgos N, Maire A, et al (2022) Automatic quality control of brain T1-weighted magnetic resonance images for a clinical data warehouse. Med Image Anal 75:102219. https://doi.org/10.1016/j.media.2021.102219
Tang L, Hui Y, Yang H, et al (2022) Medical image fusion quality assessment based on conditional generative adversarial network. Front Neurosci 16. https://doi.org/10.3389/FNINS.2022.986153/FULL
Hann E, Popescu IA, Zhang Q, et al (2021) Deep neural network ensemble for on-the-fly quality control-driven segmentation of cardiac MRI T1 mapping. Med Image Anal 71:102029. https://doi.org/10.1016/j.media.2021.102029
Aprea F, Marrone S, Sansone C (2021) Neural Machine Registration for Motion Correction in Breast DCE-MRI. In: 2020 25th International Conference on Pattern Recognition (ICPR). pp 4332–4339
Samani ZR, Alappatt JA, Parker D, et al (2020) QC-Automator: Deep Learning-Based Automated Quality Control for Diffusion MR Images. Front Neurosci 13. https://doi.org/10.3389/fnins.2019.01456
Kiser K, Meheissen MAM, Mohamed ASR, et al (2019) Prospective quantitative quality assurance and deformation estimation of MRI-CT image registration in simulation of head and neck radiotherapy patients. Clin Transl Radiat Oncol 18:120–127. https://doi.org/10.1016/j.ctro.2019.04.018
Shoroshov G, Senyukova O, Semenov D, Sharova D (2022) MRI Quality Control Algorithm Based on Image Analysis Using Convolutional and Recurrent Neural Networks. Proc IEEE Symp Comput Based Med Syst 2022-July:412–415. https://doi.org/10.1109/CBMS55023.2022.00080
Klapwijk ET, van de Kamp F, van der Meulen M, et al (2019) Qoala-T: A supervised-learning tool for quality control of FreeSurfer segmented MRI data. Neuroimage 189:116–129. https://doi.org/10.1016/j.neuroimage.2019.01.014
Islam KT, Wijewickrema S, O’Leary S (2021) A deep learning based framework for the registration of three dimensional multi-modal medical images of the head. Scientific Reports 2021 11:1 11:1–13. https://doi.org/10.1038/s41598-021-81044-7
Sokooti H, de Vos B, Berendsen F, et al (2019) 3D Convolutional Neural Networks Image Registration Based on Efficient Supervised Learning from Artificial Deformations. https://doi.org/10.48550/arxiv.1908.10235
Dubost F, de Bruijne M, Nardin M, et al (2020) Multi-atlas image registration of clinical data with automated quality assessment using ventricle segmentation. Med Image Anal 63:101698. https://doi.org/10.1016/J.MEDIA.2020.101698
Benhajali Y, Badhwar AP, Spiers H, et al (2020) A Standardized Protocol for Efficient and Reliable Quality Control of Brain Registration in Functional MRI Studies. Front Neuroinform 14. https://doi.org/10.3389/FNINF.2020.00007
Fonov VS, Dadar M, ADNI TPARG, Collins DL (2022) DARQ: Deep learning of quality control for stereotaxic registration of human brain MRI to the T1w MNI-ICBM 152 template. Neuroimage 257:. https://doi.org/10.1016/J.NEUROIMAGE.2022.119266
Ravi D, Barkhof F, Alexander DC, et al (2022) An efficient semi-supervised quality control system trained using physics-based MRI-artefact generators and adversarial training
Galib SM, Lee HK, Guy CL, et al (2020) A fast and scalable method for quality assurance of deformable image registration on lung CT scans using convolutional neural networks. Med Phys 47:99–109. https://doi.org/10.1002/mp.13890
Sokooti H, Yousefi S, Elmahdy MS, et al (2021) Hierarchical Prediction of Registration Misalignment Using a Convolutional LSTM: Application to Chest CT Scans. IEEE Access 9:62008–62020. https://doi.org/10.1109/ACCESS.2021.3074124
Muenzing SEA, van Ginneken B, Murphy K, Pluim JPW (2012) Supervised quality assessment of medical image registration: Application to intra-patient CT lung registration. Med Image Anal 16:1521–1531. https://doi.org/10.1016/j.media.2012.06.010
Koenig LN, Day GS, Salter A, et al (2020) Select Atrophied Regions in Alzheimer disease (SARA): An improved volumetric model for identifying Alzheimer disease dementia. Neuroimage Clin 26:102248. https://doi.org/10.1016/J.NICL.2020.102248
Gorgolewski K, Burns CD, Madison C, et al (2011) Nipype: A flexible, lightweight and extensible neuroimaging data processing framework in Python. Front Neuroinform 5:13. https://doi.org/10.3389/FNINF.2011.00013/ABSTRACT
Smith SM, Jenkinson M, Woolrich MW, et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1: https://doi.org/10.1016/J.NEUROIMAGE.2004.07.051
Tustison NJ, Avants BB, Cook PA, et al (2010) N4ITK: Improved N3 Bias Correction. IEEE Trans Med Imaging 29:1310–1320. https://doi.org/10.1109/TMI.2010.2046908
Baur C, Denner S, Wiestler B, et al (2021) Autoencoders for unsupervised anomaly segmentation in brain MR images: A comparative study. Med Image Anal 69:101952. https://doi.org/10.1016/J.MEDIA.2020.101952
Chatterjee S, Sciarra A, Dünnwald M, et al (2022) StRegA: Unsupervised anomaly detection in brain MRIs using a compact context-encoding variational autoencoder. Comput Biol Med 149:106093. https://doi.org/10.1016/J.COMPBIOMED.2022.106093
Luo G, Xie W, Gao R, et al (2023) Unsupervised anomaly detection in brain MRI: Learning abstract distribution from massive healthy brains. Comput Biol Med 154:106610. https://doi.org/10.1016/J.COMPBIOMED.2023.106610
Kingma DP, Ba JL (2014) Adam: A Method for Stochastic Optimization. 3rd International Conference on Learning Representations, ICLR 2015 - Conference Track Proceedings. https://doi.org/10.48550/arxiv.1412.6980
Le NQK, Ho QT, Nguyen VN, Chang JS (2022) BERT-Promoter: An improved sequence-based predictor of DNA promoter using BERT pre-trained model and SHAP feature selection. Comput Biol Chem 99:107732. https://doi.org/10.1016/J.COMPBIOLCHEM.2022.107732
Lam LHT, Do DT, Diep DTN, et al (2022) Molecular subtype classification of low-grade gliomas using magnetic resonance imaging-based radiomics and machine learning. NMR Biomed 35:e4792. https://doi.org/10.1002/NBM.4792
Gondara L (2016) Medical Image Denoising Using Convolutional Denoising Autoencoders. In: 2016 IEEE 16th International Conference on Data Mining Workshops (ICDMW). pp 241–246
Pintelas E, Livieris IE, Pintelas PE (2021) A Convolutional Autoencoder Topology for Classification in High-Dimensional Noisy Image Datasets. Sensors 21. https://doi.org/10.3390/s21227731
Acknowledgements
We would like to thank HCP-YA, ABIDE I, IXI, OASIS4, and “Food and Brain” maintenance team for providing the datasets for this study.
Author information
Authors and Affiliations
Contributions
VSGT contributed with conceptualisation, formal analysis, investigation, data curation, software, validation, and writing original draft. NKF contributed with conceptualization and supervision. ST contributed with conceptualization, methodology, editing, data curation, and supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval and Consent to Participate
This research study was conducted retrospectively using human subject data made available in open access. Hence, written informed consent is not required.
Competing Interests
A patent was filed and published on this presented work (Indian Patent, application number: 202241065452, Published on 15/11/2022).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thadikemalla, V.S.G., Focke, N.K. & Tummala, S. A 3D Sparse Autoencoder for Fully Automated Quality Control of Affine Registrations in Big Data Brain MRI Studies. J Digit Imaging. Inform. med. 37, 412–427 (2024). https://doi.org/10.1007/s10278-023-00933-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10278-023-00933-7