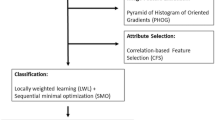
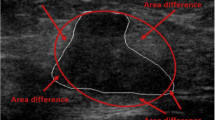
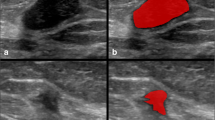
Abstract
An automated computer-aided approach might aid radiologists in diagnosing breast cancer at a primary stage. This study proposes a novel decision support system to classify breast tumors into benign and malignant based on clinically important features, using ultrasound images. Nine handcrafted features, which align with the clinical markers used by radiologists, are extracted from the region of interest (ROI) of ultrasound images. To validate that these elected clinical markers have a significant impact on predicting the benign and malignant classes, ten machine learning (ML) models are experimented with resulting in test accuracies in the range of 96 to 99%. In addition, four feature selection techniques are explored where two features are eliminated according to the feature ranking score of each feature selection method. The Random Forest classifier is trained with the resultant four feature sets. Results indicate that even when eliminating only two features, the performance of the model is reduced for each feature selection technique. These experiments validate the efficiency and effectiveness of the clinically important features. To develop the decision support system, a probability density function (PDF) graph is generated for each feature in order to find a threshold range to distinguish benign and malignant tumors. Based on the threshold range of particular features, a decision support system is developed in such a way that if at least eight out of nine features are within the threshold range, the image will be denoted as true predicted. With this algorithm, a test accuracy of 99.38% and an F1 Score of 99.05% is achieved, which means that our decision support system outperforms all the previously trained ML models. Moreover, after calculating individual class-based test accuracies, for the benign class, a test accuracy of 99.31% has been attained where only three benign instances are misclassified out of 437 instances, and for the malignant class, a test accuracy of 99.52% has been attained where only one malignant instance is misclassified out of 210 instances. This system is robust, time-effective, and reliable as the radiologists’ criteria are followed and may aid specialists in making a diagnosis.








Similar content being viewed by others
Data Availability
This study utilizes dataset of breast ultrasound images from Data in Brief [23]. The dataset is publicly available.
References
Gouzerh F, Bessiere J-M, Ujvari B, Thomas F, Dujon AM, Dormont L. Odors and cancer: Current status and future directions. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2022;1877(1):188644.
Ragab DA, Sharkas M, Marshall S, Ren J. Breast cancer detection using deep convolutional neural networks and support vector machines. PeerJ. 2019;7:e6201.
Rafid ARH, Azam S, Montaha S, Karim A, Fahim KU, Hasan MZ. An effective ensemble machine learning approach to classify breast cancer based on feature selection and lesion segmentation using preprocessed mammograms. Biology. 2022;11(11):1654.
Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA: A Cancer Journal for Clinicians. 2022;72(6):524–41.
Montaha S, Azam S, Rafid AKMRH, et al. BreastNet18: a high accuracy fine-tuned VGG16 model evaluated using ablation study for diagnosing breast cancer from enhanced mammography images. Biology. 2021;10(12):1347.
Hiatt RA, Brody JG. Environmental determinants of breast cancer. Annual review of public health. 2018;39:113-33.
Kantelhardt E, Wienke A, Addissie A, Walter Bruchhausen BA, Gregor Seliger H. Breast cancer and endocrine therapy adherence in Ethiopia: Diagnosis, treatment, and breast [Internet]. Uni-halle.de. [cited 2023 Sep 10]. Available from: https://opendata.uni-halle.de/bitstream/1981185920/103673/1/Dissertation_MLU_2023_GetachewKelboreSefonias.pdf
Dubey AK, Gupta U, Jain S. Breast cancer statistics and prediction methodology: a systematic review and analysis. Asian Pacific journal of cancer prevention. 2015;16(10):4237-45.
Na SP, Houserkovaa D. The role of various modalities in breast imaging. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151(2):209-18.
Wiacek A, Oluyemi E, Myers K, Mullen L, Bell MAL. Coherence-based beamforming increases the diagnostic certainty of distinguishing fluid from solid masses in breast ultrasound exams. Ultrasound in Medicine & Biology. 2020;46(6):1380-94.
Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499-507.
Zeimarani B, Costa MGF, Nurani NZ, Bianco SR, Pereira WCDA, Costa Filho CFF. Breast lesion classification in ultrasound images using deep convolutional neural network. IEEE Access. 2020;8:133349-59.
Masud M, Eldin Rashed AE, Hossain MS. Convolutional neural network-based models for diagnosis of breast cancer. Neural Computing and Applications. 2020:1–12.
Hassanien MA, Singh VK, Puig D, Abdel-Nasser M. Predicting breast tumor malignancy using deep convnext radiomics and quality-based score pooling in ultrasound sequences. Diagnostics. 2022;12(5):1053.
Zhuang Z, Ding W, Zhuang S, et al. Tumor classification in automated breast ultrasound (ABUS) based on a modified extracting feature network. Computerized Medical Imaging and Graphics. 2021;90:101925.
Vigil N, Barry M, Amini A, et al. Dual-intended deep learning model for breast cancer diagnosis in ultrasound imaging. Cancers. 2022;14(11):2663.
Zhuang Z, Yang Z, Raj ANJ, Wei C, Jin P, Zhuang S. Breast ultrasound tumor image classification using image decomposition and fusion based on adaptive multi-model spatial feature fusion. Computer Methods and Programs in Biomedicine. 2021;208:106221.
Pourasad Y, Zarouri E, Salemizadeh Parizi M, Salih Mohammed A. Presentation of novel architecture for diagnosis and identifying breast cancer location based on ultrasound images using machine learning. Diagnostics. 2021;11(10):1870.
Yap MH, Pons G, Marti J, et al. Automated breast ultrasound lesions detection using convolutional neural networks. IEEE Journal of Biomedical and Health Informatics. 2017;22(4):1218-26.
Ayana G, Park J, Jeong J-W, Choe S-w. A novel multistage transfer learning for ultrasound breast cancer image classification. Diagnostics. 2022;12(1):135.
Liao W-X, He P, Hao J, et al. Automatic identification of breast ultrasound image based on supervised block-based region segmentation algorithm and features combination migration deep learning model. IEEE Journal of Biomedical and Health Informatics. 2019;24(4):984-93.
Sapate SG, Mahajan A, Talbar SN, Sable N, Desai S, Thakur M. Radiomics based detection and characterization of suspicious lesions on full field digital mammograms. Computer Methods and Programs in Biomedicine. 2018;163:1-20.
Al-Dhabyani W, Gomaa M, Khaled H, Fahmy A. Dataset of breast ultrasound images. Data Brief 28, 104863 (2020). 2019.
Cai S, Wang H, Zhang X, et al. Superb microvascular imaging technology can improve the diagnostic efficiency of the BI-RADS system. Frontiers in Oncology. 2021;11:634752.
Huang Y, Han L, Dou H, Luo H, Yuan Z, Liu Q, et al. Two-stage CNNs for computerized BI-RADS categorization in breast ultrasound images. Biomedical Engineering Online. 2019;18(1):1-18.
Dobruch-Sobczak K, Piotrzkowska-Wróblewska H, Roszkowska-Purska K, Nowicki A, Jakubowski W. Usefulness of combined BI-RADS analysis and Nakagami statistics of ultrasound echoes in the diagnosis of breast lesions. Clinical Radiology. 2017;72(4):339. e7-. e15.
Srivastava V, Purwar RK. Classification of CT scan images of lungs using deep convolutional neural network with external shape-based features. Journal of Digital Imaging. 2020;33:252-61.
Liu Y, Ren L, Cao X, Tong Y. Breast tumors recognition based on edge feature extraction using support vector machine. Biomedical Signal Processing and Control. 2020;58:101825.
Khan IU, Raiaan MAK, Fatema K, et al. A computer-aided diagnostic system to identify diabetic retinopathy, utilizing a modified compact convolutional transformer and low-resolution images to reduce computation time. Biomedicines. 2023;11(6):1566.
Raiaan MAK, Al Mamun A, Islam MA, Ali ME, Mukta MSH, editors. Envy prediction from users’ photos using convolutional neural networks. 2023 International Conference on Computer, Electrical & Communication Engineering (ICCECE); 2023: IEEE.
Montaha S, Azam S, Rafid ARH, Hasan MZ, Karim A, Islam A. Timedistributed-cnn-lstm: A hybrid approach combining cnn and lstm to classify brain tumor on 3d mri scans performing ablation study. IEEE Access. 2022;10:60039-59.
Fahad NM, Sakib S, Raiaan MAK, Mukta MSH, editors. SkinNet-8: An Efficient CNN Architecture for Classifying Skin Cancer on an Imbalanced Dataset. 2023 International Conference on Electrical, Computer and Communication Engineering (ECCE); 2023: IEEE.
Montaha S, Azam S, Rafid ARH, et al. MNet-10: A robust shallow convolutional neural network model performing ablation study on medical images assessing the effectiveness of applying optimal data augmentation technique. Frontiers in Medicine. 2022;9.
Raiaan MAK, Fatema K, Khan IU, et al. A Lightweight Robust Deep Learning Model Gained High Accuracy in Classifying a Wide Range of Diabetic Retinopathy Images. IEEE Access. 2023.
Funding
This research does not include any external funding.
Author information
Authors and Affiliations
Contributions
Methodology: Sami Azam, Sidratul Montaha, Mohaimenul Azam Khan Raiaan; conceptualization: Sami Azam, Sidratul Montaha, Mohaimenul Azam Khan Raiaan, A.K.M. Rakibul Haque Rafid; formal analysis and investigation: Sami Azam, Sidratul Montaha, Mohaimenul Azam Khan Raiaan, A.K.M. Rakibul Haque Rafid; writing—original draft preparation: Sami Azam, Sidratul Montaha, Mohaimenul Azam Khan Raiaan; writing—review, and editing: Mirjam Jonkman, Md. Saddam Hossain Mukta; supervision: Sami Azam, Md. Saddam Hossain Mukta.
Corresponding author
Ethics declarations
Ethics Approval
The authors declare that no human or animal subjects have been involved in this study. Therefore, this declaration is “not applicable.”
Consent to Participate
The authors declare that no human or animal subjects have been involved in this study. Therefore, this declaration is “not applicable.”
Consent to Publish
The authors confirm that this study does not include human or animal subjects. Therefore, this declaration is “not applicable.”
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azam, S., Montaha, S., Raiaan, M.A.K. et al. An Automated Decision Support System to Analyze Malignancy Patterns of Breast Masses Employing Medically Relevant Features of Ultrasound Images. J Digit Imaging. Inform. med. 37, 45–59 (2024). https://doi.org/10.1007/s10278-023-00925-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10278-023-00925-7