Abstract
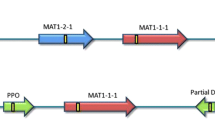
Mating-type genes were cloned and sequenced in the heterothallic phytopathogenic fungus Exserohilum monoceras, a candidate bioherbicide for the control of Echinochloa weeds in rice fields. Using PCR-based methods, we determined both idiomorphs and flanking regions of these genes. The structural organization of the E. monoceras Mat locus was similar to that of other heterothallic ascomycetes. The MAT1-1-1 gene was 1191 bp and encoded a predicted protein of 379 amino acids with an α box domain. The MAT1-2-1 gene was 1093 bp and encoded a predicted protein of 345 amino acids with a high mobility group box domain. Expression of both MAT genes was detected under vegetative and invasive growth conditions. MAT-specific primers were developed to assess the mating-type frequency of E. monoceras field isolates. Both mating types were observed in Japanese field isolates. Analysis of mating types and sexual hybridization of E. monoceras could provide useful approaches for the conventional genetic manipulation of this fungus to produce a more efficient bioherbicide.






Similar content being viewed by others
References
Alcorn JL (1978) Setosphaeria monoceras sp. nov., ascigeneous state of Exserohilum monoceras. Mycotaxon 7:411–414
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Arie T, Kaneko I, Yoshida T, Noguchi M, Nomura Y, Yamaguchi I (2000) Mating-type genes from asexual phytopathogenic ascomycetes Fusarium oxysporum and Alternaria alternata. Mol Plant Microbe Interact 13:1330–1339
Bennett RS, Yun SH, Lee TY, Turgeon BG, Arseniuk E, Cunfer BM, Bergstrom GC (2003) Identity and conservation of mating type genes in geographically diverse isolates of Phaeosphaeria nodorum. Fungal Genet Biol 40:25–37
Charudattan R (2001) Biological control of weeds by means of plant pathogens: significance for integrated weed management in modern agro-ecology. Biocontrol 46:229–260
Charudattan R (2005) Use of plant pathogens as bioherbicides to manage weeds in horticultural crops. Proc Fla State Hortic Soc 118:208–214
Cozijnsen AJ, Howlett BJ (2003) Characterisation of the mating-type locus of the plant pathogenic ascomycete Leptosphaeria maculans. Curr Genet 43:351–357
Gafur A, Tanaka C, Ouchi S, Tsuda M (1997) A PCR-based method for mating type determination in Cochliobolus heterostrophus. Mycoscience 38:455–458
Holm LG, Plucknett DL, Pancho JV, Herberger JP (1977) The world’s worst weeds: distribution and biology. The University Press of Hawaii, Honolulu
Izumitsu K, Yoshimi A, Kubo D, Morita A, Saitoh Y, Tanaka C (2009) The MAPKK kinase ChSte11 regulates sexual/asexual development, melanization, pathogenicity, and adaptation to oxidative stress in Cochliobolus heterostrophus. Curr Genet 55:439–448
Kronstad JW, Staben C (1997) Mating type in filamentous fungi. Annu Rev Genet 31:245–276
Leubener-Metzger G, Horwitz BA, Yoder OC, Turgeon BG (1997) Transcripts at the mating type locus of Cochliobolus heterostrophus. Mol Gen Genet 256:661–673
Milgroom MG (1996) Recombination and the multilocus structure of fungal populations. Annu Rev Phytopathol 34:457–477
Morita A, Saitoh Y, Izumitsu K, Tanaka C (2011) Teleomorph formation Setosphaeria monoceras, a perfect state of Exserohilum monoceras, by Japanese isolates. Mycoscience. doi:10.1007/s10267-011-0140-5
Nelson MA (1996) Mating systems in ascomycetes: a romp in the sac. Trends Genet 12:69–74
Ochman H, Gerber AS, Hartl DL (1988) Genetic applications of an inverse polymerase chain reaction. Genetics 120:621–623
O’Gorman CM, Fuller HT, Dyer PS (2009) Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457:471–474
Paoletti M, Rydholm C, Schwier EU, Anderson MJ, Szakacs G, Lutzoni F, Debeaupuis JP, Latgé JP, Denning DW, Dyer PS (2005) Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Curr Biol 15:1242–1248
Pöggeler S (2002) Genomic evidence for mating abilities in the asexual pathogen Aspergillus fumigatus. Curr Genet 42:153–160
Punt PJ, Dingemanse MA, Jacobs-Meijsing BJM, Pouwels PH, van den Hondel CAMJJ (1988) Isolation and characterization of the glyceraldehyde-3-phosphate dehydrogenase gene of Aspergillus nidulans. Gene 69:49–57
Saitoh Y, Izumitsu K, Morita A, Shimizu K, Tanaka C (2010) ChMCO1 of Cochliobolus heterostrophus is a new class of metallo-oxidase, playing an important role in DHN-melanization. Mycoscience 51:327–336
Sharon A, Yamaguchi K, Christiansen S, Horwitz BA, Yoder OC, Turgeon BG (1996) An asexual fungus has the potential for sexual development. Mol Gen Genet 251:60–68
Tanaka C, Kubo Y, Tsuda M (1991) Genetic analysis and characterization of Cochliobolus heterostrophus colour mutants. Mycol Res 95:49–56
Tanaka E, Kumagawa T, Tanaka C, Koga H (2011) Simple transformation of the rice false smut fungus Villosiclava virens by electroporation of intact conidia. Mycoscience. doi: 10.1007/s10267-011-0115-6
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tredway LP, Stevenson K, Burpee LL (2003) Mating type distribution and fertility status in Magnaporthe grisea populations from turfgrasses in Georgia. Plant Dis 87:435–441
Tsukamoto H, Gohbara M, Tsuda M, Fujimori T (1997) Evaluation of fungal pathogens as biological control agents for the paddy weed, Echinochloa species by drop inoculation. Ann Phytopathol Soc Jpn 63:366–372
Turgeon BG (1998) Application of mating type gene technology to problems in fungal biology. Annu Rev Phytopathol 36:115–137
Turgeon BG, Bohlmann H, Ciuffetti LM, Christiansen SK, Yang G, Schäfer W, Yoder OC (1993) Cloning and analysis of the mating type genes from Cochliobolus heterostrophus. Mol Gen Genet 238:270–284
Van Wert SL, Yoder OC (1992) Structure of the Cochliobolus heterostrophus glyceraldehyde-3-phosphate dehydrogenase gene. Curr Genet 22:29–35
Wirsel S, Horwitz B, Yamaguchi K, Yoder OC, Turgeon BG (1998) Single mating type-specific genes and their 3′UTRs control mating and fertility in Cochliobolus heterostrophus. Mol Gen Genet 259:272–281
Yoshimi A, Tsuda M, Tanaka C (2004) Cloning and characterization of the histidine kinase gene Dic1 from Cochliobolus heterostrophus that confers dicarboximide resistance and osmotic adaptation. Mol Genet Genomics 271:228–236
Zdobnov EM, Apweiler R (2001) InterProScan—an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17:847–848
Zhang W, Watson AK (1997) Efficacy of Exserohilum monoceras for the control of Echinochloa species in rice (Oryza sativa). Weed Sci 45:144–150
Acknowledgments
We thank Drs. M. Tsuda (Prof. Emeritus, Kyoto Univ.) and H. Tsukamoto (Japan Tobacco Inc. Plant Innovation Center) for providing some of the E. monoceras strains and Dr. Y. Yamasue (Kyoto Univ.) for providing Echinochloa oryzicola seeds used in this study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Morita, A., Saitoh, Y., Izumitsu, K. et al. Molecular organization of the mating type (Mat) locus of Exserohilum monoceras (Setosphaeria monoceras), a bioherbicide agent for Echinochloa weeds. Mycoscience 53, 92–101 (2012). https://doi.org/10.1007/s10267-011-0141-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10267-011-0141-4