Abstract
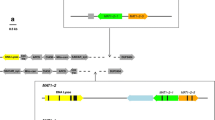
To understand the organization of the mating type locus of Stagonosporopsis tanaceti and Stagonosporopsis chrysanthemi, and its potential role in the epidemiology of ray blight of pyrethrum and chrysanthemum, respectively, the mating type (MAT) locus of these species was cloned and characterized using PCR-based techniques. The complete MAT locus of each species was cloned and annotated including complete and/or partial hypothetical genes flanking the idiomorphs. Analysis of the MAT locus organization indicated that S. chrysanthemi is likely homothallic with both MAT1-2-1 and MAT1-1-1 co-located within the idiomorph, and this was supported by production of the teleomorph in cultures of single-conidial-derived isolates. Sequencing of the MAT locus and flanking genes of S. tanaceti demonstrated that only a single MAT gene, MAT1-1-1, was located within this idiomorph and suggesting that S. tanaceti is heterothallic. MAT-specific PCR primers were developed and used to determine mating type of isolates sampled from diseased pyrethrum fields in Australia. These results indicated that only one mating type of S. tanaceti was present in Tasmania, Australia. The absence of a second mating type suggests that this species does not reproduce sexually in Tasmania, Australia and that ascospores are unlikely to be a source of inoculum for ray blight of pyrethrum. The MAT-specific PCR assay will be a valuable tool to distinguish mating types present among isolates of S. tanaceti, to monitor populations of S. tanaceti for the introduction of a second mating type and to differentiate S. tanaceti from S. chrysanthemi.



Similar content being viewed by others
References
Arie T, Christiansen SK, Yoder OC, Turgeon BG (1997) Efficient cloning of ascomycete mating type genes by PCR amplification of the conserved MAT HMG box. Fungal Genet Biol 21:118–130
Aveskamp MM, de Gruyter J, Woudenberg JHC, Verkley GJM, Crous PW (2010) Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol 65:1–60
Barve MP, Arie T, Salimath SS, Muehlbauer FJ, Peever TL (2003) Cloning and characterization of the mating type (MAT) locus from Ascochyta rabiei (teleomorph: didymella rabiei) and a MAT phylogeny of legume-associated Ascochyta spp. Fungal Genet Biol 39:151–167
Cherif M, Chilvers MI, Akamatsu H, Kaiser WJ, Peever TL (2006) Cloning of the mating type locus from Ascochyta lentis (teleomorph : didymella lentis) and development of a multiplex PCR mating assay for Ascochyta species. Curr Genet 50:203–215
Chesters CG, Blakeman JP (1967) Host range and variation in virulence of Mycosphaerella ligulicola. Ann Appl Biol 60:385–390
Chilvers MI, Peever TL, Akamatsu H, Chen WD, Muehlbauer FJ (2007) Didymella rabiei primary inoculum release from chickpea debris in relation to weather variables in the Pacific northwest of the United States. Can J Plant Pathol 29:365–371
Chilvers MI, Rogers JD, Dugan FM, Stewart JE, Chen WD, Peever TL (2009) Didymella pisi sp. nov., the teleomorph of Ascochyta pisi. Mycol Res 113:391–400
Chilvers MI, Jones S, Pethybridge S, Peever TL (2011) Evolution of mating type genes: heterothallism is ancestral in the Didymellaceae North Central American Phytopathological Society. Omaha, NE
Debuchy R, Berteaux-Lecellier V, Silar P (2010) Mating systems and sexual morphogenesis in ascomycetes. Cell Mol Biol Filament Fungi: 501–535
Furukawa T, Kishi K (2002) Production of perithecia of various ascomycotina on water agar medium emended with leaf pieces. J Phytopathol–Phytopathol Z 150:625–628
Hernandez-Bello MA, Chilvers MI, Akamatsu H, Peever TL (2006) Host specificity of Ascochyta spp. infecting legumes of the Viciae and Cicerae tribes and pathogenicity of an interspecific hybrid. Phytopathology 96:1148–1156
Jones S (2010) Characterization of cultural, biological and molecular variability of Phoma ligulicola isolates associated with ray blight disease of pyrethrum and chrysanthemum. PhD Thesis. University of Tasmania, Australia
Kaiser WJ (1997) Inter- and intra-national spread of ascochyta pathogens of chickpea, faba bean, and lentil. Can J Plant Pathol 19:215–224
Liu Y, Wittier RF (1995) Thermal asymetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25:674–681
McCoy RE, Horst RK, Dimock AW (1972) Environmental factors regulating sexual and asexual reproduction by Mycosphaerella ligulicola. Phytopathology 62:1188–1195
Metzenberg RL, Glass NL (1990) Mating type and mating strategies in Neurospora. Bio Essays 12:53–59
Peever TL, Salimath SS, Su G, Kaiser WJ, Muehlbauer FJ (2004) Historical and contemporary multilocus population structure of Ascochyta rabiei (teleomorph : didymella rabiei) in the Pacific northwest of the United States. Mol Ecol 13:291–309
Pethybridge SJ, Hay FS (2001) Influence of Phoma ligulicola on yield, and site factors on disease development, in Tasmanian pyrethrum crops. Australas Plant Path 30:17–20
Pethybridge SJ, Hay F, Jones S, Wilson C, Groom T (2006) Seedborne infection of pyrethrum by Phoma ligulicola. Plant Dis 90:891–897
Pethybridge SJ, Hay FS, Clarkson RA, Groom T, Wilson CR (2008a) Host range of Australian Phoma ligulicola var. inoxydablis isolates from pyrethrum. J Phytopathol 156:506–508
Pethybridge SJ, Hay FS, Esker PD, Gent DH, Wilson CR, Groom T, Nutter FW (2008b) Diseases of pyrethrum in Tasmania: challenges and prospects for management. Plant Dis 92:1260–1272
Pethybridge SJ, Scott JB, Hay FS (2012) Lack of evidence for recombination or spatial structure in Phoma ligulicola var. inoxydabilis populations from Australian pyrethrum fields. Plant Dis 96:746–751
Stevens FL (1907) The chrysanthemum ray blight. Bot Gaz 44:0241–0258
Trapero-Casas A, Kaiser WJ (1992) Development of Didymella rabiei, the teleomorph of Ascochyta rabiei, on chickpea straw. Phytopathology 82:1261–1266
Trapero-Casas A, Navas-Cortes JA, Jimenez-Diaz RM (1996) Airborne ascospores of Didymella rabiei as a major primary inoculum for Ascochyta blight epidemics in chickpea crops in southern Spain. Eur J Plant Pathol 102:237–245
Turgeon BG, Yoder OC (2000) Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genet Biol 31:1–5
Vaghefi N, Pethybridge SJ, Ford R, Nicolas ME, Crous PW, Taylor PWJ (2012) Stagonosporopsis spp. associated with ray blight disease of Asteraceae. Australas Plant Path 41:675–686
Van Der Aa HA, Noordeloos ME, Degruyter J (1990) Species concepts in some larger genera of the coelomycetes. Stud Mycol 32:3–19
Wilson AD, Kaiser WJ (1995) Cytology and genetics of sexual compatibility in Didymella rabiei. Mycologia 87:795–804
Woudenberg JHC, De Gruyter J, Crous PW, Zwiers LH (2012) Analysis of the mating-type loci of co-occurring and phylogenetically related species of Ascochyta and Phoma. Mol Plant Pathol 13:350–362
Acknowledgments
This project was partially supported by Michigan State University’s Project GREEEN (Grant number GR09-093) and Lyman Briggs College, Undergraduate Research Grant; and Botanical Resources Australia—Agricultural Services Pty. Ltd. We would like to thank Dr. Quan Zeng of Michigan State University for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann.
Rights and permissions
About this article
Cite this article
Chilvers, M.I., Jones, S., Meleca, J. et al. Characterization of mating type genes supports the hypothesis that Stagonosporopsis chrysanthemi is homothallic and provides evidence that Stagonosporopsis tanaceti is heterothallic. Curr Genet 60, 295–302 (2014). https://doi.org/10.1007/s00294-014-0435-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-014-0435-0