Abstract
Odontogenic infections (OIs) occasionally spread to deep facial and neck tissues. Our study aimed to explore the role of Streptococcus anginous group (SAG) in these severe OIs. A retrospective study of patients aged ≥ 18 years who required hospital care for acute OI was conducted. We analysed data of OI microbial samples and recorded findings of SAG and other pathogens. These findings were compared with data regarding patients’ prehospital status and variables of infection severity. In total, 290 patients were included in the analyses. The most common (49%) bacterial finding was SAG. Other common findings were Streptococcus viridans and Prevotella species, Parvimonas micra, and Fusobacterium nucleatum. Infection severity variables were strongly associated with SAG occurrence. Treatment in an intensive care unit was significantly more common in patients with SAG than in patients without SAG (p < 0.001). In addition, SAG patients expressed higher levels of C-reactive protein (p = 0.001) and white blood cell counts (p < 0.001), and their hospital stays were longer than those of non-SAG patients (p = 0.001). SAG is a typical finding in severe OIs. Clinical features of SAG-related OIs are more challenging than in other OIs. Early detection of SAG, followed by comprehensive infection care with prompt and careful surgical treatment, is necessary due to the aggressive behaviour of this dangerous pathogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Odontogenic infections (OIs) can spread deeper into the surrounding tissues, requiring hospitalization, and potentially posing a life-threatening risk [1,2,3]. Behind more severe deep neck infections lie certain predictors, such as psychiatric disorder, alcohol abuse, and diabetes mellitus [4,5,6], although previously healthy patients may require hospital care due to an OI, as well [2, 7]. Mandibular molars are the most common sources of severe OIs [8, 9], and these arise from apical periodontitis in particular [10]. In addition, deep infection may occur after tooth removal to treat acute or subacute local infection symptoms [11]. Early management of the dental infection focus by tooth removal or root canal treatment effectively reduces dispersion of the infection, however, occasionally, the pathogenic microbes invade deeper spaces, causing infection complications such as pneumonia, septicaemia, and endocarditis [12,13,14].
The complexity of the oral microbiome is well known, with over 500–700 bacterial species identified [15,16,17]. Both cariogenic and periodontal pathogens have been detected in heart valve specimens, suggesting a causative relationship between dental infections and cardiovascular diseases [18, 19]. OIs and dental infectious diseases in general are polymicrobial and often caused by anaerobic and facultative bacteria [20, 21]. Streptococcus viridans species, including Streptococcus anginosus group (SAG)—formerly and popularly known as Streptococcus milleri group, have been identified as the most frequent bacteria in head and neck infections of odontogenic origin, resulting in increasing concern for antibiotic resistance, particularly for penicillin and clindamycin [22,23,24]. This emphasizes the importance of timely local treatment of the dental focus [5, 25].
Members of the SAG—Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus—share common traits regarding clinical associations but differ slightly in their abscess formation capacity [26]. Recent and earlier findings suggest Streptococcus anginosus group as a key factor in odontogenic descending necrotizing mediastinitis, pulmonary infections, and brain abscesses [27,28,29], while the presence of SAG has also been reported in many other morbidities such as skin and soft tissue infections, genitourinary infections and liver abscesses [30, 31]. However, the SAG organisms are categorized as commensals in the oropharynx as well as in the gastrointestinal and genitourinary tracts [32].
The aim of our study was to clarify the occurrence of SAG in deep OIs and to evaluate the clinical features and severity of these infections in relation to OIs caused by other microbes. Our hypothesis was that infections caused by SAG are associated with more complicated clinical features.
Patients and methods
Study design
Electronic health records of all acute maxillofacial infection patients treated at the Töölö Hospital Emergency Department between the years 2015 and 2019 were retrospectively reviewed, and data for study variables were retrieved from electronic patient records of each patient.
Inclusion and exclusion criteria
Patients aged ≥ 18 years who required treatment and hospital stay for acute and deep OI (i.e., abscess or cellulitis of facial or neck region of dental origin) were included in the present study. All infections were confirmed as odontogenic by oral and maxillofacial surgeons. Patients with infection of unknown origin or other than odontogenic focus as a reason for maxillofacial infection were excluded from the analyses. Patients without any microbial finding in bacterial culture were also excluded.
Study variables
Occurrence of SAG and other microbiological findings were recorded based on the routine bacterial culture reports released from Helsinki University Hospital Laboratory Services (HUSLAB). Patient’s prehospital and infection severity variables were compared between patients with and without SAG findings.
Age, sex, current smoking, excess alcohol consumption or regular use of drugs, and history of immunosuppressive disease or medications, or both, and duration of symptoms prior to hospital care were considered in the analyses as prehospital variables. Excess alcohol consumption was considered to be ≥ 12 doses per week for women and ≥ 23 doses for men; one alcohol dose was 12 g of pure alcohol.
To evaluate infection severity, need for treatment in an intensive care unit (ICU), level of C-reactive protein (CRP), white blood cell (WBC) count and tympanic body temperature at hospital admission, length of hospital stay (LOHS) in an ICU and in hospital, and occurrence of a distant infection (i.e., different distant infections and infection complications) were analysed. Multivariate analyses were conducted for need for ICU treatment, LOHS, and CRP at hospital admission.
Additionally, types of specific infection complications and distant infections were reported.
Statistical analysis
IBM SPSS for Macintosh (version 27.0, IBM Corp., Armonk, NY, USA) software package was used for statistical analyses. Categorical variables were cross-tabulated and analysed with Pearson’s Chi-squared test or Fisher’s exact test if expected values were below 5. Student’s t-test and Mann–Whitney U test were used to compare differences between study groups in continuous variables. Pairwise comparisons were performed as post hoc analyses for Pearson’s Chi-squared test using Z test and Dunn’s (1964) procedure for Kruskal–Wallis H test, both with a Bonferroni correction for multiple comparisons. Treatment in ICU, LOHS, and CRP level were separately selected as outcome variables for binomial logistic regression analysis; age, sex, current smoking, excess alcohol consumption or regular use of drugs, history of immunosuppressive disease or medication, or both, origin of infection in mandible, and SAG-positive microbial sample were selected as independent variables. LOHS and CRP level were dichotomized by group median, and age was categorized into tertiles. P values ˂ 0.05 were considered significant throughout the study.
Results
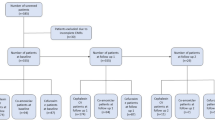
Data from altogether 357 patients with OI were collected from the electronic patient records. Of these patients, a bacterial sample had been obtained and microbial growth reported for 290 patients, who subsequently formed the final study population.
In 194 subjects (67%), the bacterial culture finding was a mixed finding of aerobic and anaerobic bacteria. Aerobic bacteria alone were reported in 60 cultures (21%) and anaerobic bacteria alone in 55 cultures (19%). The most common bacterial finding was SAG, which was found in 49% of patients. In 123 cases (42%), SAG was diagnosed with anaerobic bacteria. Other common findings were normal oral microbial flora (48%) and anaerobic Gram-negative rods (47%, Fig. 1). Among other common findings were Streptococcus viridans group and Prevotella species, Parvimonas micra, and Fusobacterium nucleatum.
In only few samples, findings not representative of normal oral flora were discovered. Among these were the aerobic cocci betahaemolytic streptococci (n = 4; 1%) and Staphylococcus aureus (n = 4; 1%), aerobic Gram-negative rods Klebsiella pneumoniae (n = 3; 1%) and Enterobacter cloacae (n = 3; 1%), and anaerobic species of the Bacteroides fragilis group (n = 3; 1%).
Of prehospital variables, mandibular odontogenic infection focus was more strongly associated with SAG than focus in the maxilla (p = 0.012, Table 1). Additionally, there was a significant association between SAG and tooth removal before hospitalization (p = 0.041). Antibiotic treatment was administered to all but one patient. In most patients (n = 269, 93%), metronidazole was combined with cephalosporin or penicillin. Clindamycin was administered to 8 patients (3%). In 35 patients (12%), antibiotic treatment was altered during hospital stay according to clinical and microbiological findings.
Infection severity variables were strongly associated with SAG occurrence. ICU treatment was significantly more common in patients with SAG than in patients without SAG (p < 0.001). Compared with non-SAG patients, SAG patients expressed higher levels of CRP (p < 0.001) and WBC counts (p = 0.001), and their LOHS was longer (p = 0.001), as presented in Table 2.
Distant infections and/or other infection complications occurred in 22 of 290 patients (8%, Table 2). Complications were more common in patients with SAG than in other patients (n = 14, 64% vs. n = 8, 36% of infection complications), however, the difference was not statistically significant (p = 0.153). Bacterial blood culture sample was collected and analysed in 125 patients. Of the 12 patients with septicaemia, 10 were SAG-positive and two were SAG-negative (p = 0.042). Other distant infections and/or other infection complications in 13 patients were pneumonia (n = 10), endocarditis (n = 2), necrotizing fasciitis (n = 2), urosepsis (n = 1), and embolic renal infarction (n = 1); of these patients, 8 (62%) were SAG-positive and 5 (38%) SAG-negative (p = 0.354). Three of the patients died; all were SAG-positive.
According to binomial logistic regression analyses (Table 3), patients with a SAG-positive microbial sample were 3.4 times more likely to need treatment in an ICU (p < 0.001). Smoking (odds ratio, OR = 2.1, p = 0.024) and origin of OI in mandible (OR = 5.8, p = 0.021) added independently to the odds for need for ICU treatment. Odds for longer hospital stay were 2.3-fold (p < 0.001), for higher CRP values 1.9-fold (p < 0.010), and for higher than median WBC counts 1.7-fold (p = 0.0351) for SAG-positive patients relative to SAG-negative patients.
Discussion
We examined the occurrence of SAG in deep OIs and the clinical features and severity of these infections in relation to OIs caused by other microbes. Our hypothesis that infections caused by SAG are associated with more complicated clinical features was confirmed.
SAG was present in nearly half of the patients, and it was the most common bacterial finding. SAG-associated infections were significantly more complicated than those without SAG. Need for ICU treatment was significantly more likely for SAG-positive patients (OR = 3.4, p < 0.001). Higher levels of CRP, WBC, and LOHS were associated with SAG. These findings imply that SAG infections are complicated, severe, and life-threatening. The typical nature of SAG-associated OIs differs from OIs caused by other pathogens.
In principle, SAG organisms are classified as commensals. However, it is essential to emphasize the clinical importance of the aggressive behaviour of SAG in severe OIs. SAG bacteria are able to cause infections if they gain access through mucosa to sterile sites, i.e., the underlying tissue or blood, and can, therefore, be viewed as opportunistic pathogens. It has previously been shown that SAG-related OIs are linked to the most severe disease, with difficulties in swallowing and opening the mouth [33]. In spreading OIs, SAG behaviour is known to be typically complicated and abscess-forming [34]. Our findings confirm the clinical significance of this aggressive pathogen (Fig. 2). However, despite the clinically notable typical infection features, the true pathogenic potential of SAG remains to be elucidated [35]. A recent study by Ismail et al. [36] clarified SAG occurrence in different infections in children and adolescents and discovered that S. intermedius was more commonly present in head and neck infections than other SAG species. Thus, differences exist in infection locations and SAG species.
A 40-year-old male smoker without underlying diseases had had toothache in the right lower jaw for 3 days before onset of significant swelling and fever (39.2 °C). At hospital admission, C-reactive protein level was 253 mg/L and white blood cell count was 18.2 109/L. The patient underwent three wide surgical interventions to subside the deep oral, facial, and neck infection. Bacterial culture from the pus sample showed Streptococcus anginosus and mixed anaerobic flora. Blood culture was also positive (anaerobic Gram-positive coccus and anaerobic Gram-negative rod). A. Dental panoramic tomography radiograph showed the right mandibular third molar with periapical lesion as the main infection focus. B. Computer tomography imaging at hospital admission demonstrated bilateral gas formation in neck tissues from the level of skull base to the upper mediastinal space at the right side. The airway was also deviated. C. Extensive neck approaches were required, including temporal and upper mediastinal drainages. Swelling and tightness in buccal and periorbital areas remained significant after the third surgery. The overall length of hospital stay was 32 days. The patient eventually recovered well from the infection. A written consent was obtained from the patient for use of the image
A suggested novel trait in the pathogenicity of S. anginosus may be its ability to produce bacteriocin designated as Angicin, which enhances membrane permeabilization [37], inhibits the growth of closely related species [38], and enables compartmental abscess formation. Acid tolerance exhibited by the micro-organism is another factor that may facilitate chronic inflammation and abscess development [39]. Ardent fever, chills, and systemic toxaemia have been observed in severe SAG infections; upper airway obstructions and necrotizing pneumonia are possible outcomes of abscess formation [33, 40]. Our results show that in SAG-related OI patients the airways are more often compromised, the infection parameters reflect a more severe infection, and the treatment of these infections takes longer than in other OIs. The potentially fatal characteristics of SAG infections emphasize the importance of early detection of these OIs.
To identify SAG infections without unnecessary delay their clinical features should be recognized. These include rapid and aggressive progression. According to our study, high CRP and WBC level as well as fever at hospital admission are typical for SAG infections. Gas bubble formation in radiological imaging is indicative of SAG [27]. The surgeon should consider SAG at an early stage and treat these infections with precision. Sufficient surgical treatment with wide approaches and careful abscess drainage is necessary to halt the progression of SAG infection. SAG is typically identified in abscesses as part of polymicrobial infections with anaerobes. In our study, SAG was identified concomitantly with anaerobic bacteria in 85% of the samples with SAG. This underscores the need for abscess drainage as a crucial component of successful treatment. Furthermore, the simultaneous removal of odontogenic infection focus is known to shorten LOHS compared with tooth extraction after initial infection treatment [25]. Compartmental, tissue-disrespectful progression is typical of SAG, thus, close monitoring of the patient and, if necessary, repeated evaluation of the abscess chambers is essential.
Compared with figures from previous reports [13, 41], infection complications and distant infections were relatively scarce, occurring in 8.3% of patients. The most common of these were septicaemia and pneumonia, whereas necrotizing fasciitis and endocarditis were each observed in only two patients. The rare occurrence of the most severe infections highlights the typical pattern of OIs; even deep infections requiring intensive care are limited to the neck area if treatment is effective and started in time. On the other hand, SAG is often associated with infection complications and distant infections, especially with septicaemia. All three deceased patients here were SAG-positive. These findings underscore the life-threatening features of SAG in OI patients.
Our research shows a clear association between the site of infection and the presence of SAG; OIs originating from the mandible occurred significantly more often in conjunction with SAG than maxillary infections. In general, most severe OIs are known to originate more often from the lower molars [7, 9]. We also observed that tooth removal prior to hospitalization was significantly more common in patients with SAG than those without (56% vs. 44%, p = 0.041). The finding suggests that previous tissue damage may predispose to the development of severe SAG-associated OIs.
The importance of immune defense and previous diseases is also worth considering. Currently, however, there are no reports showing that microbial findings would be altered in severe OIs compared to milder infections according to patients’ systemic condition, although the proportion of patients with underlying systemic disease has been reported to have increased [2, 41]. Our earlier studies have shown that severe OIs occur most often in previously healthy adults [11, 42]. Dental procedures including extraction and root canal treatment disrupt the mucosal barrier allowing introduction of mucosal opportunistic bacteria to normally sterile tissue, are local oral risk factors for severe OIs [10, 11]. Furthermore, ineffective early treatment of OI may increase the risk of a severe OI [42]. The findings of the present study are in line with these previous results as immunosuppression was not associated with SAG finding (Table 1). Additionally, only 18% of patients had underlying immunosuppressive medication or disease. In conclusion, the risk of these infections seems to be primarily associated with local factors such as dental treatment procedures, delayed or inadequate dental treatment, or increased systemic susceptibility to infection complications, rather than altered microbial flora in certain systemic conditions.
In addition to drainage and treatment of odontogenic focus, antimicrobial treatment is often required for successful treatment of purulent OIs. These infections are usually polymicrobial, consisting primarily of SAG or other Viridans streptococci and anaerobic bacteria. Other microbes are rare findings, as observed also in our study. Yet another important aspect to consider in choosing the antimicrobial is the local resistance situation. Fortunately, in Finland, SAG and other Viridans streptococci have remained fairly sensitive to penicillin, with only a 1–5% resistance rate, and oral anaerobes remain highly susceptible to metronidazole [43]. In contrast, resistance to clindamycin of oral streptococci has recently slightly increased and needs to be monitored. Therefore, the Finnish Current Care Guideline recommends primarily using penicillin for oral streptococci and metronidazole for anaerobic bacteria, which may be beta-lactamase producers [44]. Broader spectrum cephalosporins or clindamycin are recommended instead of penicillin in case of penicillin allergy. If metronidazole is contraindicated, clindamycin can be combined with penicillin to cover anaerobes, if needed. If the above combinations are contraindicated, broad-spectrum amoxicillin-clavulanic acid may be used as monotherapy.
For the retrospective study design, some of the patients was excluded from the study because of unavailable bacterial cultures. In addition, PCR testing to determine the species of bacteria is not performed in our unit routinely, thus, the determination of bacteria was based on culture only. Data of patients treated in primary or secondary health care was unavailable; our data were collected from hospitalized patients. A prospective study design would refine these parameters. New and effective treatments for SAG-related infections should be explored in future studies.
Conclusions
SAG is a typical finding in severe OIs. Clinical features of SAG-related OIs are more aggressive than in other OIs. Early detection of SAG, followed by comprehensive infection care with prompt and careful surgical treatment, is essential due to the aggressive behaviour of this stealthy pathogen.
Data availability
The datasets generated in the study are not publicly available as this requires research ethics approval and participant consent. A summary of final findings will be available from the corresponding author on reasonable request.
References
Green AW, Flower EA, New NE. Mortality associated with odontogenic infection! Br Dent J. 2001;190(10):529–30.
Seppanen L, Lauhio A, Lindqvist C, et al. Analysis of systemic and local odontogenic infection complications requiring hospital care. J Infect. 2008;57(2):116–22.
Fu B, McGowan K, Sun JH, et al. Increasing frequency and severity of odontogenic infection requiring hospital admission and surgical management. Br J Oral Maxillofac Surg. 2020;58(4):409–15.
Pham Dang N, Delbet-Dupas C, Mulliez A, et al. Five Predictors Affecting the Prognosis of Patients with Severe Odontogenic Infections. Int J Environ Res Public Health. 2020;17(23):8917.
Jundt JS, Gutta R. Characteristics and cost impact of severe odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(5):558–66.
Huang TT, Tseng FY, Liu TC, et al. Deep neck infection in diabetic patients: comparison of clinical picture and outcomes with nondiabetic patients. Otolaryngol Head Neck Surg. 2005;132(6):943–7.
Furuholm J, Rautaporras N, Uittamo J, et al. Health status in patients hospitalised for severe odontogenic infections. Acta Odontol Scand. 2021;27:1–7.
Seppanen L, Lemberg KK, Lauhio A, et al. Is dental treatment of an infected tooth a risk factor for locally invasive spread of infection? J Oral Maxillofac Surg. 2011;69(4):986–93.
Alotaibi N, Cloutier L, Khaldoun E, et al. Criteria for admission of odontogenic infections at high risk of deep neck space infection. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132(5):261–4.
Furuholm J, Uittamo J, Rautaporras N, et al. Are there differences between dental diseases leading to severe odontogenic infections requiring hospitalization? A retrospective study. Quintessence Int. 2022. https://doi.org/10.3290/j.qi.b2793183.
Rautaporras N, Uittamo J, Furuholm J, et al. Severe infections after teeth removal - are we doing enough in preventing them? J Clin Exp Dent. 2022;14(3):e254–62.
Rautaporras N, Furuholm J, Uittamo J, et al. Deep odontogenic infections-identifying risk factors for nosocomial pneumonia. Clin Oral Invest. 2021;25(4):1925–32.
Weise H, Naros A, Weise C, et al. Severe odontogenic infections with septic progress - a constant and increasing challenge: a retrospective analysis. BMC Oral Health. 2019;19(1):173.
Strom BL, Abrutyn E, Berlin JA, et al. Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Ann Intern Med. 1998;129(10):761–9.
Paster BJ, Boches SK, Galvin JL, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183(12):3770–83.
Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721–32.
Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192(19):5002–17.
Nakano K, Inaba H, Nomura R, et al. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J Clin Microbiol. 2006;44(9):3313–7.
Nakano K, Nemoto H, Nomura R, et al. Detection of oral bacteria in cardiovascular specimens. Oral Microbiol Immunol. 2009;24(1):64–8.
Brook I, Frazier EH, Gher ME. Aerobic and anaerobic microbiology of periapical abscess. Oral Microbiol Immunol. 1991;6(2):123–5.
Sundqvist G. Ecology of the root canal flora. J Endod. 1992;18(9):427–30.
Heim N, Jurgensen B, Kramer FJ, et al. Mapping the microbiological diversity of odontogenic abscess: are we using the right drugs? Clin Oral Invest. 2021;25(1):187–93.
Flynn TR, Shanti RM, Levi MH, et al. Severe odontogenic infections, part 1: prospective report. J Oral Maxillofac Surg. 2006;64(7):1093–103.
Rega AJ, Aziz SR, Ziccardi VB. Microbiology and antibiotic sensitivities of head and neck space infections of odontogenic origin. J Oral Maxillofac Surg. 2006;64(9):1377–80.
Heim N, Warwas FB, Wiedemeyer V, et al. The role of immediate versus secondary removal of the odontogenic focus in treatment of deep head and neck space infections. A retrospective analysis of 248 patients. Clinical Oral Investiga. 2019;23(7):2921–7.
Claridge JE 3rd, Attorri S, Musher DM, et al. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis. 2001;32(10):1511–5.
Sakai T, Sano A, Azuma Y, et al. Streptococcus anginosus group infection as a predictor for the progression of descending necrotizing mediastinitis. Ann Palliat Med. 2021;10(4):4008–16.
Marks PV, Patel KS, Mee EW. Multiple brain abscesses secondary to dental caries and severe periodontal disease. Br J Oral Maxillofac Surg. 1988;26(3):244–7.
Shinzato T, Saito A. The Streptococcus milleri group as a cause of pulmonary infections. Clin Infect Dis. 1995;21(Suppl 3):S238–43.
Al Majid F, Aldrees A, Barry M, et al. Streptococcus anginosus group infections: Management and outcome at a tertiary care hospital. J Infect Public Health. 2020;13(11):1749–54.
Morii K, Fujiwara S, Nakamura S, et al. Streptococcus Anginosus Group-associated Pyogenic Liver Abscess. Intern Med. 2018;57(15):2271–2.
Fazili T, Riddell S, Kiska D, et al. Streptococcus anginosus Group Bacterial Infections. Am J Med Sci. 2017;354(3):257–61.
Jiang S, Li M, Fu T, et al. Clinical Characteristics of Infections Caused by Streptococcus Anginosus Group. Sci Rep. 2020;10(1):9032.
Han JK, Kerschner JE. Streptococcus milleri: an organism for head and neck infections and abscess. Arch Otolaryngol Head Neck Surg. 2001;127(6):650–4.
Asam D, Spellerberg B. Molecular pathogenicity of Streptococcus anginosus. Mol Oral Microbiol. 2014;29(4):145–55.
Ismail K, Hughes I, Moloney S, et al. Streptococcus anginosus group infections in hospitalised children and young people. J Paediatr Child Health. 2021. https://doi.org/10.1111/jpc.15840.
Vogel V, Bauer R, Mauerer S, et al. Angicin, a novel bacteriocin of Streptococcus anginosus. Sci Rep. 2021;11(1):24377.
Vogel V, Olari LR, Jachmann M, et al. The bacteriocin Angicin interferes with bacterial membrane integrity through interaction with the mannose phosphotransferase system. Front Microbiol. 2022;13: 991145.
Sasaki M, Kodama Y, Shimoyama Y, et al. Aciduricity and acid tolerance mechanisms of Streptococcus anginosus. J Gen Appl Microbiol. 2018;64(4):174–9.
Saha BK. Rapidly progressive necrotizing pneumonia: remember the Streptococcus anginosus group! Pan Afr Med J. 2020;36:116.
Velhonoja J, Laaveri M, Soukka T, et al. Deep neck space infections: an upward trend and changing characteristics. Eur Arch Otorhinolaryngol. 2020;277(3):863–72.
Uittamo J, Lofgren M, Hirvikangas R, et al. Severe odontogenic infections: focus on more effective early treatment. Br J Oral Maxillofac Surg. 2020;58(6):675–80.
Heikkinen T, Järvinen A, Meri S, et al. Mikrobiologia (Microbiology). Finnish Medical Society Duodecim. Available online at: https://www.oppiportti.fi/op/opk04495; 2020
Antimicrobials in acute dentistry. Current Care Guidelines. Working group set up by the Finnish Medical Society Duodecim. Helsinki: The Finnish Medical Society Duodecim, 2022 (referred May 26, 2022). Available online at: www.kaypahoito.fi.
Acknowledgements
This research was supported by the authors’ institutions and did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital.
Author information
Authors and Affiliations
Contributions
JS drafted the manuscript and developed the study design. NR and JU scrutinized the patient records and collected the crude data. JF performed statistical analysis and prepared the manuscript. HV grouped microbes and completed the setup. All authors were involved in revising the manuscript and provided intellectual input, reading, and approving the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest regarding this work.
Ethical approval
The study protocol was approved by the Internal Review Board of the Head and Neck Centre, Helsinki University Hospital, Helsinki, Finland (HUS/58/2020). The study was conducted in adherence to the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Furuholm, J., Uittamo, J., Rautaporras, N. et al. Streptococcus anginosus: a stealthy villain in deep odontogenic abscesses. Odontology 111, 522–530 (2023). https://doi.org/10.1007/s10266-022-00763-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-022-00763-z