Abstract
Due to their reduced morphology, non-photosynthetic plants have been one of the most challenging groups to delimit to species level. The mycoheterotrophic genus Monotropastrum, with the monotypic species M. humile, has been a particularly taxonomically challenging group, owing to its highly reduced vegetative and root morphology. Using integrative species delimitation, we have focused on Japanese Monotropastrum, with a special focus on an unknown taxon with rosy pink petals and sepals. We investigated its flowering phenology, morphology, molecular identity, and associated fungi. Detailed morphological investigation has indicated that it can be distinguished from M. humile by its rosy pink tepals and sepals that are generally more numerous, elliptic, and constantly appressed to the petals throughout its flowering period, and by its obscure root balls that are unified with the surrounding soil, with root tips that hardly protrude. Based on genome-wide single-nucleotide polymorphisms, molecular data has provided clear genetic differentiation between this unknown taxon and M. humile. Monotropastrum humile and this taxon are associated with different Russula lineages, even when they are sympatric. Based on this multifaceted evidence, we describe this unknown taxon as the new species M. kirishimense. Assortative mating resulting from phenological differences has likely contributed to the persistent sympatry between these two species, with distinct mycorrhizal specificity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The subfamily Monotropoideae (Ericaceae), is distributed throughout the Northern Hemisphere (Bidartondo and Bruns 2001; Kron et al. 2002). It is characterized by its achlorophyllous and fully mycoheterotrophic habit, with scale-like leaves, bisexual and actinomorphic flowers, free sepals and petals, and a superior 1–6 locule ovary (Wallace 1987; Wu et al. 2016). As currently circumscribed, Monotropoideae contains ca. 15 species in 12 genera: Eremotropa Andres, Hypopitys Hill, Allotropa Torr. & A.Gray, Hemitomes A.Gray, Monotropa L., Monotropastrum Andres, Monotropsis Schwein., Pityopus Small, Pleuricospora A.Gray, Pterospora Nutt., Cheilotheca Hook.f., and Sarcodes Torr. (Rose and Freudenstein 2014; Wallace 1975; Wu et al. 2016; Zhao et al. 2019). Although its center of species diversity is western North America, with seven endemic species, at least five species, including Monotropastrum humile (D.Don) Hara, three Cheilotheca species, and Eremotropa sciaphila Andres, are Asian endemics (Wallace 1975; Wu et al. 2016; Zhao et al. 2019).
The monotypic genus Monotropastrum, including M. humile, is widely distributed in East Asia, from the Himalayas to Japan (POWO 2022). However, the taxonomic treatment of Monotropastrum is still confusing in terms of varietal recognition and generic attribution (Tsukaya et al. 2008). Although in some cases it has been separated into two varieties, namely M. humile var. humile and M. humile var. glaberrimum Hara (Hara 1961, 1965), recognition of M. humile var. glaberrimum has often been neglected (POWO 2022; Qin and Wallace 2005). Consequently, M. humile has often been recognized as having considerable morphological variation (Qin and Wallace 2005).
The generic classification of Monotropastrum is also confusing. Monotropastrum shares ovary and fruit characteristics with Cheilotheca, including unilocular ovaries with parietal placentation and baccate fruits (Andres 1935, 1936). This has led to Monotropastrum often being considered synonymous with Cheilotheca (Hsu et al. 1998; Keng 1974; Keng and Hsieh 1978). Therefore, Keng and Hsieh (1978) transferred M. humile and M. humile var. glaberrimum to Cheilotheca humilis (D.Don) H.Keng and C. humilis var. glaberrimum (H.Hara) H.Keng & Hsieh, respectively. However, despite their similarities, there are some substantial differences between Monotropastrum and Cheilotheca, such as the petals lacking or having a thickened apex (Wallace 1987). Considering the genetic distance between Monotropastrum and Cheilotheca is greater than that between other genera in the Monotropoideae, Monotropastrum and Monotropa (Tsukaya et al. 2008), we highlight that Monotropastrum and Cheilotheca should be accepted, with M. humile var. glaberrimum transferred into Cheilotheca, as proposed by Tsukaya et al. (2008).
Regarding the taxonomic treatment of Monotropastrum, it is also noteworthy that an unknown Monotropastrum taxon, with rosy pink petals and sepals, has long been recognized around Kirishima, Kagoshima Prefecture, Japan (Imamura and Kurogi 2003). It is morphologically similar morphologically to M. humile f. humile in having nodding flowers at anthesis, petals without a thickened apex, a single-loculed ovary with parietal placentation, and baccate fruits. Consequently, it has tentatively been treated as a color variant of M. humile, known as M. humile f. roseum Honda (Imamura and Kurogi 2003). However, the flowering seasons for this taxon and M. humile do not overlap (Kurogi, unpublished data), and their mycorrhizal morphology and root systems differ considerably (Imamura and Kurogi 2003). Therefore, this unknown taxon may be a cryptic species rather than a color variant. Organisms with reduced morphology have always presented a challenge for systematists because of the relative paucity of characters (Barrett and Freudenstein 2011). Non-photosynthetic plants with highly reduced leaves are, therefore, prime candidates for being cryptic species (Barrett and Freudenstein 2011; de Vega et al. 2008; Thorogood et al. 2008). Morphological changes accompanying the shift to mycoheterotrophy include the loss of the leaf laminae, and reduced underground organs. Therefore, it is crucial to examine multiple characteristics for species delimitation in mycoheterotrophic species (Barrett and Freudenstein 2011).
Here, we have applied an integrative taxonomic approach to test whether this taxon should be considered a distinct species from Monotropastrum humile. We investigated discontinuities in flowering phenology and floral morphology that could uniquely diagnose this taxon. We then reconstructed the phylogenetic relationships to examine whether the taxon represents distinct evolutionary lineages, based on MIG-seq [multiplexed inter-simple sequence repeat (ISSR) genotyping by sequencing] data. Finally, given that fungal-associate identity may be relevant in delimiting mycoheterotrophic taxa (Barrett and Freudenstein 2011; Barrett et al. 2022; Freudenstein and Barrett 2014), we investigated the mycorrhizal fungal communities of this taxon and M. humile, including at a sympatric site, using high-throughput DNA sequencing. Our multifaceted evidence leads us to conclude that this taxon is morphologically, phenologically, phylogenetically, and ecologically distinct, and should, therefore, be recognized as a separate species. Consequently, we have described it as a new species, M. kirishimense Suetsugu. Our data are consistent with a study that has shown that fungal host utilization enhances species delimitation in mycoheterotrophic orchids (Freudenstein and Barrett 2014). Our study presents the exciting possibility that a host shift in M. kirishimense, toward a specific Russula lineage, triggered ecological speciation.
Materials and methods
Specimen collection and preservation
We collected 50 Monotropastrum kirishimense plants encompassing ten Japanese populations. A total of 38 individuals of M. humile, including five M. humile f. roseum plants, were collected throughout Japan and Taiwan from a total of eight populations, as shown in Table S1. For comparative study, we also collected one specimen from Vietnam [Hsu 10691 (STG00764), hereafter referred to as Monotropastrum sp. 1]. This specimen differs morphologically from typical M. humile, and has glabrous flowers and broad, somewhat ridged fruits (Fig. S1). To minimize disturbance to the local populations, the minimum number of samples required for molecular analysis were collected. However, at least one voucher specimen encompassing the entire plant was deposited in KYO, MZ, TAIF, TI and TNS representing each population. Scale leaves for DNA analysis were immediately dried using silica gel and stored at room temperature until DNA extraction, while 1–3 root fragments (ca. 1 mm in diameter and 3–5 mm in length) were collected from each specimen for molecular barcoding of mycorrhizal fungi. Each root sample was transferred to a 1.5 mL tube containing 99.5% ethanol, and stored at − 20 °C. The herbarium acronyms follow Index Herbariorum (Thiers 2021).
Morphological observation
We compared the morphological characters of Monotropastrum kirishimense, M. humile, and Monotropastrum sp. 1, using the samples listed in Table S1. The morphological variation in M. kirishimense and M. humile was also investigated by reviewing the literature and herbarium specimens (at KYO, TAIF and TI) from other localities. The morphological characters were visually observed under a stereomicroscope and measured using a digital caliper. We note that M. kirishimense is somewhat similar to M. humile f. roseum described from Sadogashima, Niigata Prefecture, Japan, and has rosy pink flowers. Therefore, we examined the M. humile f. roseum type specimen at TI (TI00205063) in detail, and additional M. humile f. roseum specimens from other localities, to identify consistent morphological differences between M. kirishimense and M. humile.
Flowering phenology analysis
Field observations of the flowering phenology from a sympatric site were used to determine whether differences in the flowering phenology play a role in maintaining reproductive integrity between Monotropastrum kirishimense and M. humile. For quantitative comparison, we counted the scapes of M. kirishimense and M. humile from the Onami population (31° 55′ N 130° 50′ E), where both species occur sympatrically, between April 26 and July 22, 2003, and between May 6 and July 17, 2004. They were classified into four developmental stages: (A) emerging (the aboveground organs becoming visible through the leaf litter), (B) flowering (anthers and stigma becoming visible from the perianth tube), (C) wilting (with blackened tepals), and (D) fruiting (ovary becoming large and protruding from the dried out tepals). These stages were counted manually while walking along a fixed route of ca. 500 m, at intervals of approximately 3 weeks.
High-throughput plant phylogenetic analysis
A phylogenetic tree of Monotropastrum plants was constructed based on MIG-seq, which encompasses microsatellite-associated reduced-representation DNA sequencing with restriction site-associated DNA sequencing (RAD-seq) (Suyama and Matsuki 2015). After extracting the genomic DNA from the silica-dried samples using the cetyltrimethylammonium bromide (CTAB) method, we prepared an MIG-seq library, as per Suetsugu et al. (2021b) and Suyama et al. (2022). This included 32 M. kirishimense samples from seven populations, 19 M. humile samples from eight populations, including two M. humile f. roseum individuals, and one Monotropastrum sp. 1 sample (Table S1). The library was sequenced using an Illumina MiSeq Sequencer (Illumina, San Diego, CA, USA) with a MiSeq Reagent Kit v. 3 (150 cycle, Illumina). The raw MIG-seq data were deposited in the DDBJ Sequence Read Archive (DRA, accession number DRA014598).
After removing the primer regions and low-quality sequencing reads (Suetsugu et al. 2021b), 7,014,511 reads (137,539 ± 7234 reads per sample) were obtained from the original 7,804,056 raw reads (153,021 ± 7863 per sample). The Stacks v. 2.60 pipeline was used for de novo single-nucleotide polymorphism (SNP) discovery (Rochette et al. 2019). The following parameters were used: minimum depth of coverage required to create a stack (m) = 3, maximum distance allowed between the stacks (M) = 2, number of mismatches allowed between the sample loci when building the catalog (n) = 2. Only SNPs retained by 26 or more samples were extracted, and SNPs with high heterozygosity (Ho ≥ 0.6) were removed. Moreover, SNP sites with fewer than three minor alleles were filtered out. Finally, 1000 SNPs from 543 loci were provided for the subsequent analyses. SNP-based maximum likelihood (ML) phylogeny was inferred using RAxML v. 8.2.10 (Stamatakis 2014), with a GTR substitution model with Lewis’ ascertainment bias correction and 1000 bootstrap replicates.
Molecular analysis of the mycorrhizal fungi
Genomic DNA was extracted from the root tips of 10 Monotropastrum kirishimense plants from four populations (Table S1), and 23 M. humile plants (including two M. humile f. roseum plants) from five populations, using CTAB methods. We amplified the ITS region of the mycorrhizal fungi using the primer set ITS86F/ITS4 (Waud et al. 2014) fused with 3–6-mer Ns and with the Illumina forward/reverse sequencing primer. To add the Illumina sequencing adapters, supplemental PCR was also performed as described in Suetsugu et al. (2021a, b). Equal volumes of each PCR amplicon were pooled and purified using the AMPure XP Kit (Beckman Coulter, CA, USA). The sequencing libraries were processed in an Illumina MiSeq sequencer, with the MiSeq Reagent Micro Kit v. 2 (300 cycles, Illumina, USA). The sequence data were deposited in the DRA (accession number DRA013047).
After sequencing, we performed bioinformatic analysis using Claident v. 0.2.2019.05.10 (Tanabe and Toju 2013), as described in Suetsugu and Matsubayashi (2021). Erroneous sequence reads were removed based on the CD-HIT-OTU method (Li et al. 2012), using the clcleanseqv command in Claident. The remaining sequencing reads were clustered into operational taxonomic units (OTUs) at a 97% threshold similarity, using VSEARCH v. 2.8.0 (Rognes et al. 2016). The OTUs were subjected to de novo and reference-based chimera removal, based on the UCHIME algorithm (Nilsson et al. 2019). The OTU taxonomic assignment was performed based on the query-centric auto-k-nearest-neighbor (QCauto) and the lowest common ancestor (LCA) algorithms (Tanabe and Toju 2013). The functional guild for each fungal OTU was estimated using the FUNGuild database (Nguyen et al. 2016). In subsequent analyses, we used the taxa designated as ectomycorrhizal fungi by FUNGuild, because all monotropoid species obtain their carbohydrates from mycorrhizal fungi that link them to the surrounding trees, on which the fungi form ectomycorrhizae (Bidartondo and Bruns 2001, 2002; Matsuda et al. 2011; Yokoyama et al. 2005).
Because all the Monotropastrum kirishimense and M. humile plants were predominantly colonized by OTUs assigned to Russula, we downloaded several Russula sequences closely related to the OTUs detected here, based on BLAST searches, from the International Nucleotide Sequence Database Collaboration (INSDC) database. The sequences obtained were aligned using ClustalW in MEGA X (Kumar et al. 2018). The aligned sequences were then used to reconstruct phylogenetic relationships using MEGA X (Kumar et al. 2018) with ML analysis, with a GTR + I + G model and 1000 bootstrap replicates (lnL = − 3108.04). Our infrageneric classification of Russula follows Shimono et al. (2004), Looney et al. (2018), Wang et al. (2019), and Buyck et al. (2020).
Results
Morphological characters
Review and analysis of herbarium specimens, protologues, and living plants revealed few morphological characters that consistently differed between Monotropastrum kirishimense and M. humile (Figs. 1, 2, 3, 4, 5). Although the shape of the floral disc is recognized as a diagnostic character between M. humile and its closely related species (Tsukaya et al. 2008), the floral disc of M. kirishimense has thin protrusions elongated to bend backward (Fig. 4d), similar to that of M. humile (Tsukaya et al. 2008).
Morphological comparison of the aboveground parts of Monotropastrum kirishimense and M. humile. Monotropastrum kirishimense in a Fujieda-shi, Shizuoka Pref., b Ena-shi, Gifu Pref., and c Kirishima-shi, Kagoshima Pref. Monotropastrum humile in d Waga-gun, Iwate Pref. and e Tarumizu-shi, Kagoshima Pref., and f M. humile f. roseum in Sakyo-ku, Kyoto Pref. Arrowheads indicate spreading sepals. Scale bars: 3 cm. Photographed by Masayuki Sato (a), Katsumi Iwahori (b), Shuichi Kurogi (c), Shin Terui (d), Kazushige Uemori (e), and Kenji Suetsugu (f)
Monotropastrum humile and its monotropoid association found at the M. kirishimense type locality (on June 25, 2019, except for the flowering plants). a Flowering plants (on May 17, 2019). Arrowheads indicate spreading sepals. b Fruiting plant. c Fruiting scapes with root ball. d, e Magnification of the root ball. Root tips and branching are easily recognizable. Arrowheads indicate root tip apices. Scale bars: 3 cm (a–c), 1 cm (d), and 5 mm (e). Photographed by Hideo Shimada (a) and Kenji Suetsugu (b–e)
Monotropastrum kirishimense and its monotropoid association (holotype). a Flowering scape with root ball. b, c Flowering plants. d Flowers, top view. e, f Magnification of the root ball. Root tips are not apparent, but white fungal hyphae are visible. Arrowheads indicate the root tip apices. Scale bars: 3 cm (a–c), 1 cm (d, e), and 5 mm (f). Photographed by Kenji Suetsugu
Monotropastrum kirishimense (holotype). a Flower. b Root ball with the interpenetrating Pinus densiflora root system. c Flower with perianth removed. d Floral discs with the basal part of the filaments and protrusions from the floral disc. e Petal, adaxial view. f Petal, abaxial view. g Anther. Scale bars: 1 cm (a, b), 5 mm (c, e, f), and 2 mm (d, g). Photographed by Kumi Hamasaki
Monotropastrum kirishimense (drawn from the holotype). a Flowering scapes with root ball, with the interpenetrating Pinus densiflora root system. b Flower, side view. c Flower, top view. d Flower after the removal of perianth. e Flower after the removal of perianth and filaments. f Sepals, adaxial view. g Petal, adaxial view. h Scale leaf, adaxial view. i Floral discs and basal part of ovary. j Filaments. Scale bars: 3 cm (a), 1 cm (b, c), 5 mm (d–i), and 2 mm (j). Drawn by Kumi Hamasaki
However, Monotropastrum kirishimense can be distinguished from M. humile by its rosy pink tepals and several other features. First, the flowers of M. kirishimense usually bear 4–9 (up to 11) generally elliptic sepals that are constantly appressed to the petals throughout its flowering period, while the flowers of M. humile usually bear 2–3 (up to 5) generally oblong sepals that are usually spreading during peak anthesis (Figs. 1, 2, 3, 4, 5). Second, M. kirishimense flowers and ovaries are more rounded, with a lower aspect ratio than those of M. humile. Although the M. humile ovary also expands and becomes rounded after pollination, the flowers and ovaries of M. kirishimense are stout even before pollination (Figs. 1, 2, 3, 4, 5). Third, M. kirishimense is always shorter above ground (typically < 5 cm, vs. > 5 cm in M. humile; Figs. 2a, 3b), whereas the underground stalk is longer in M. kirishimense (typically > 10 cm, vs. < 5 cm in M, humile; Figs. 2c, 3a). More importantly, as suggested by Imamura and Kurogi (2003), the root ball morphology differs completely between these two species. The M. kirishimense root ball is obscure and unified with the surrounding soil, with little protrusion of root tips, and a white mantle indicating mycorrhizal formation (Figs. 3f, 4b, 5a). Meanwhile, that of M. humile can easily be distinguished from the soil and has relatively distinct root tips (Fig. 2e).
Although Monotropastrum humile f. roseum is also distinguished from M. humile f. humile by its red flowers, Honda (1957) did not describe its characteristics other than floral coloration. Its holotype comprises only the aboveground parts. Nonetheless, due to the spreading oblong-elliptic to obovate-elliptic sepals, we can conclude that M. kirishimense is not conspecific with M. humile f. roseum. Additional sampling of M. humile f. roseum indicates that M. humile f. roseum cannot be distinguished from M. humile f. humile, other than in coloration. Additional sampling has also shown that M. humile f. roseum has a reddish ovary (Fig. 1f), and that M. kirishimense has rosy pink tapels (Fig. 1a–c).
Flowering phenology
Monotropastrum humile flowers mature much earlier than those of M. kirishimense (Fig. 6). On 19 May 2003, all the M. humile plants were in bloom or had already begun to wilt, whereas no M. kirishimense plants were visible. On 30 June 2003, many M. humile plants had already disappeared, with the few remaining individuals all being at the fruiting stage. Meanwhile, most of the M. kirishimense individuals were flowering. These M. humile and M. kirishimense plants reached almost equivalent flowering stages on 19 May 2003 and 30 June 2003, respectively, indicating that M. humile flowers ca. 40 days before M. kirishimense.
Plant phylogeny
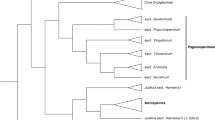
The Monotropastrum ML phylogenetic tree separated M. kirishimense from the remaining taxa, and the monophyly of each clade was highly supported (a 100% bootstrap value; Fig. 7). Although Monotropastrum sp. 1 is not morphologically identical to M. humile, because of its glabrous flower organs and broad, somewhat ridged fruits, it was embedded within a clade comprising the remaining M. humile. The genetic differentiation between Monotropastrum sp. 1 and M. humile was not as large as that between M. kirishimense and M. humile. Therefore, it might be appropriate to consider it an intraspecific variant of M. humile. However, considering that Monotropastrum sp. 1 is distinguished from M. humile by many morphological traits, further investigation would help to determine whether there are cryptic Monotropastrum species other than M. kirishimense within the species complex.
Fungal community
Of the 93 fungal operational taxonomic units (OTUs) (204,485 sequencing reads) retrieved using the fungal ITS primer set, 25 OTUs (185,571 reads; 90.8% of all reads) were considered putative ectomycorrhizal fungi (Table S2). Most of the fungal ITS sequences of the Monotropastrum kirishimense and M. humile mycobionts had high DNA-sequence homology with Russula species (seven OTUs, 182,693 reads; 89.3% of all reads, Fig. 8) (Table S2). All the M. kirishimense plants were predominantly colonized by the same Russula OTU (Russula OTU2; 32,308 sequencing reads; 84.7% of all reads). This dominant association between M. kirishimense and a single Russula OTU among multiple populations more than 700 km apart provides strong evidence that M. kirishimense exhibits specialized interactions with this OTU (Table S2, Fig. 8). Only one other Russula OTU (Russula OTU3; 12 reads) was detected, in two samples; given its extremely low number of sequencing reads, this is likely an opportunistic fungus with no fundamental role for M. kirishimense. Hence, we conclude that M. kirishimense is primarily specialized on Russula OTU2. For each of the M. humile individuals, one of five Russula OTUs was the predominant colonizer (150,373 reads; 90.4% of all reads; Table S2). However, Russula OTU2, dominant in M. kirishimense, was not detected in any of the M. humile individuals, even in the sympatric Onami population.
Our fungal ITS sequence ML phylogenetic analysis has shown that the Monotropastrum kirishimense mycobionts were clustered nearest to sequences of Russula aff. alboareolata (AB509955), belonging to the subsection Virescentinae (Fig. 9). This subsection has not been reported as a mycobiont of the 93 M. humile individuals collected in Japan, Taiwan, and China across 15 populations (Bidartondo and Bruns 2001; Matsuda et al. 2011; Min et al. 2012; Yokoyama et al. 2005). In contrast, all the Russula OTUs associated with the M. humile individuals investigated in the present study formed a clade with mycobionts previously detected in M. humile (Fig. 9).
Phylogenetic tree of partial internal transcribed spacer rDNA sequences from mycorrhizal fungi of the Monotropastrum kirishimense and M. humile plants investigated in this study (bold type) and from the International Nucleotide Sequence Database Collaboration (INSDC) database. Nodes supported by bootstrap values < 50% are not shown
Discussion
Our morphological investigation has indicated that Monotropastrum kirishimense can be distinguished from M. humile by its rosy pink tepals, more numerous elliptic sepals constantly appressed to the petals throughout its flowering period, and obscure root balls unified with the surrounding soil, with little protrusion of the root tips. Our MIG-seq-based phylogenetic tree has also shown that M. kirishimense and the other M. humile species complex can be separated into two monophyletic clades, with a 100% bootstrap value. We have therefore determined that M. kirishimense should be treated as an independent species, based on its morphological and phylogenetic distinctness.
Floral trait differentiation between taxa plays a key role in reducing interspecific pollen transfer, either through phenological isolation (a pre-mating barrier caused by differences in flowering time) or floral isolation (a pre-mating barrier caused by differences in morphological, visual, or olfactory traits) (Chapurlat et al. 2020). Given their overall similarity in floral features, the two species are unlikely to experience pollinator-mediated isolation. Indeed, we have observed that Monotropastrum kirishimense and M. humile are mainly pollinated by the bumblebee Bombus diversus (K. Suetsugu, unpublished data). Reproductive isolation between M. kirishimense and M. humile is also unrelated to the spatial separation of these species, given that they grow adjacent to each other.
Phenological isolation between plant taxa has substantial potential to lead to reproductive isolation. Monotropastrum humile flowers more than a month before M. kirishimense, with only a brief period (if any) of overlap at the end of M. humile flowering. This divergence in flowering time could be directly selected as a reinforcement mechanism to reduce hybridization before complete speciation (Osborne et al. 2020). Therefore, speciation between M. kirishimense and M. humile may be reinforced by differences in the timing of floral maturation. However, it is also possible that flowering-time divergence could be selected after speciation is complete, as a mechanism to avoid wasting reproductive effort on unfit hybrids (Hopkins 2013). Reproductive asynchrony in flowering time can reduce heterospecific pollen deposition, helping to ensure conspecific mating (Lowry et al. 2008).
Metabarcoding-based community profiling revealed that Monotropastrum kirishimense and M. humile are predominantly associated with different Russula lineages. Monotropastrum kirishimense is consistently associated with Russula OTU2 (in subsection Virescentinae), even in the Onami population, where M. humile, associated with different OTUs, grows sympatrically within a few meters. Therefore, we can conclude that their genetic characteristics drive the differences in mycorrhizal interaction between the two species. For M. kirishimense, the association with Russula OTU2 encompasses four sampling localities spanning the geographic distribution of the species. Despite extensive studies on its mycorrhizal communities based on 113 individuals collected in Japan, Taiwan, and China across 20 populations (Bidartondo and Bruns 2001; Matsuda et al. 2011; Min et al. 2012; Yokoyama et al. 2005; present study), the subsection Virescentinae has never been reported as a mycobiont of M. humile.
In contrast, all the Russula OTUs associated with M. humile individuals collected from all the five populations in our studies were closely related or identical to OTUs previously reported as mycobionts of M. humile (Bidartondo and Bruns 2001; Matsuda et al. 2011; Min et al. 2012; Yokoyama et al. 2005). Consequently, we conclude that the two species differ in mycorrhizal specificity, and that M. kirishimense has a specialized association with Russula OTU2, although more extensive assessments may reveal that some M. humile individuals are associated with Russula OTU2.
Although M. kirishimense is widely distributed in Japan, it is specialized on Russula OTU2, and is much rarer locally than M. humile, which is associated with diverse members of the Russulaceae. Although the high host specificity of M. kirishimense may contribute to its rarity, the ecology of its fungal OTU, including its preferred habitat, soil requirements, or fidelity to a specific ectomycorrhizal host tree, remains unexplored. Notably, M. kirishimense occurs only in coniferous forests (dominated primarily by Pinus densiflora), while M. humile commonly occurs in not only coniferous forests, but also other ectomycorrhizal forests, such as fagaceous forests. Russula OTU2 may be preferentially associated with P. densiflora. Further studies are needed to investigate the distribution patterns and abundance of Russula species associated with M. kirishimense and M. humile.
Our findings imply that the distinct mycorrhizal communities play a crucial role in the niche partitioning and coexistence of M. kirishimense and M. humile. Because (i) classical theoretical ecology predicts that two species competing for the same resources cannot stably coexist (Gause 1934) and (ii) mycoheterotrophic plants depend on mycorrhizal fungi for their carbon demands (Merckx 2013), divergent mycorrhizal associations can play a vital role in reducing resource competition. Previous studies have also showed that sympatric (at least initially) mycoheterotrophic plants often have distinct mycorrhizal communities and display strong spatial segregation, even if they share some fungal OTUs (Bidartondo and Bruns 2005; Jacquemyn et al. 2014; McCormick and Jacquemyn 2014; Taylor and Bruns 1999). Our findings may therefore indicate that niche differentiation via segregation of mycorrhizal fungi represents an important mechanism contributing to sympatry. Given that vertical depth partitioning among closely related ectomycorrhizal fungi is a common phenomenon (Mujic et al. 2016; Taylor et al. 2014), the different root depths of M. kirishimense and M. humile may be an adaptation to effectively exploit vertically separated fungal partners.
Furthermore, speciation between Monotropastrum kirishimense and M. humile may be partially due to resource partitioning, with specialization on different fungal hosts leading to reproductive isolation (see also Barrett and Freudenstein 2011; Barrett et al. 2022). Speciation via host shift is one of the most plausible modes of ecological speciation (Calcagno et al. 2007; Fry 2003). This scenario begins with the formation of host races comprising host-affiliated, genetically differentiated groups within the parental species (Drès and Mallet 2002; Jacquemyn et al. 2018). Disruptive selection on host-specificity, indicated by trade-offs in performance between hosts, can lead to further specialization, and promote the formation of two daughter species (Jacquemyn et al. 2018; Rundle and Nosil 2005).
Although little is known about the genetic basis of mycoheterotroph–mycorrhizal associations, the high mycorrhizal specificity observed in many mycoheterotrophs is thought to be the result of physiological fine-tuning to adapt to particular fungi (Hynson and Bruns 2009). Breakdown of coadapted gene complexes controlling host specificity may, therefore, be responsible for postzygotic isolation, in the form of reduced hybrid fitness. Hybridization between ecotypes within a single mycoheterotrophic species with different host specificity can considerably reduce progeny fitness due to a lower probability of mycoheterotrophic growth (Jacquemyn et al. 2016, 2018). Consequently, differences in mycorrhizal communities have been suggested to contribute to reproductive isolation among mycoheterotrophic plants (Barrett and Freudenstein 2011; Barrett et al. 2022; Jacquemyn et al. 2018). Future investigations, including artificial interspecific cross-pollination experiments and in-situ seed baiting, are required to determine whether mycorrhizal associations can prevent hybrid seeds from establishing successful seedlings, thus acting as a post-mating barrier in these two Monotropastrum species. We also note that genotypically distinct M. humile individuals in the different populations tended to be predominantly colonized by different Russula OTUs, highlighting potential race formation within M. humile. However, it is impossible to exclude the possibility that the local availability of Russula species is the primary determinant, because there was no geographic mixing of different genotypes. More extensive sampling across a much broader geographic range would facilitate a more robust understanding of the evolutionary dynamics of mycorrhizal specificity within M. humile.
In summary, we have shown that Monotropastrum kirishimense is distinct from M. humile based on morphology, flowering phenology, and the molecular identity of itself and its fungal partners. Phenological differences (a pre-mating reproductive barrier) and distinct mycorrhizal specificity (a post-mating reproductive barrier) are likely to contribute to the ongoing sympatry of M. kirishimense and M. humile. Mycoheterotrophic plants are often susceptible to environmental destruction, because they are highly dependent on the fungi and the trees that sustain them (Suetsugu et al. 2020). Therefore, many members of the Monotropoideae are restricted to old-growth forests and are now in danger of extinction (Min et al. 2012). The rare and previously unrecognized M. kirishimense can now receive conservation recognition for the first time. This study highlights the importance of integrative taxonomy to avoid under-assessing biodiversity.
Taxonomic treatment
Monotropastrum kirishimense Suetsugu, sp. nov. (Figs. 1a–c, 3, 4, 5)
Type. JAPAN. Kagoshima Pref, Kirishima-shi, Makizono-cho, Ohnami-ike, 25 June 2019, Kenji Suetsugu KS424 (holotype: KYO!, dried plant on an herbarium sheet and liquid-preserved material in a bottle labeled as the same specimen; isotypes: TI!, TNS!, dried plant on an herbarium sheet).
Diagnosis. Monotropastrum kirishimense is similar to M. humile but differs in its rosy pink tepals, more numerous (4–11) elliptic sepals constantly appressed to the petals throughout its flowering period, and obscure root balls unified with the surrounding soil, with little protrusion of the root tips.
Terrestrial, mycoheterotrophic herb. Root ball unified with the surrounding soil, with little protrusion of the root tips, 4.7–6.3 cm in diam; roots 0.7–0.9 mm in diam. Stems erect, 8.5–20 cm long, 3.8–7.8 mm in diam. below flower, arising in nodding position from root ball; uniflorous. Scale leaves on upper stem narrowly ovate, 16–21 mm long, 6–10 mm wide, entire to erose, apex acute to rounded, glabrous. Scale leaves at the base of stem shorter and more densely crowded on axis, glabrous. Flower campanulate, solitary, nodding at anthesis, 15.3–25 mm long, 11.1–13.9 mm wide at the middle, 10.1–17.0 mm at apex. Sepals 4–9(–11), rosy pink, elliptic, 14.2–19.8 mm long, 8.0–12.6 mm wide, appressed to petals, slightly erose, abaxially glabrous or slightly pubescent, adaxially pubescent. Petals (3–)4–5, rosy pink, obovate-oblong to cuneate-oblong, 16.8–20.3 mm long, 11.1–14.8 mm wide, entire, abaxially glabrous or slightly pubescent, adaxially densely pubescent, base broadly saccate, apex dilated. Stamens 10–14; filaments 10.8–13 mm long, pubescent; anthers yellow, horizontally reniform, 1.6–2.2 mm long, 0.9–1.6 mm wide, with a single terminal slit across connate sacs. Pollen grains monad 23–30 μm in diam., commonly triporate, pores protruding, fine verrucate–rugulate. Style 2.5–3.2 mm long, merging imperceptibly with apex of the ovary. Stigma funnel-form, blue on margin, ca. 1.5 mm long, 5–6 mm in diam. Ovary ovoid, unilocular, without distinct ridges, 9.5–15.8 mm long, 9–11.2 mm wide, glabrous; parietal placentae 10–14. Fruit white, erect to nodding, ovoid-globose, abruptly narrowed to style, 10.1–18.7 mm long, 10.6–23.5 mm wide, interior; seeds numerous, embedded within fleshy pulp. Seeds ovoid, ca. 0.4 mm long, ca. 0.2 mm wide; testa not prolonged, minutely reticulate.
Additional specimens examined (paratype). JAPAN. Kyushu District—Kagoshima Pref.: Kirishima-shi, Mt. Eboshi, 25 June 2019, Kenji Suetsugu KS426 (KYO); Kirishima-shi, Mt. Eboshi, 26 June 2010, Kenji Suetsugu Mk1 (KYO); Kirishima-shi, Makizono-cho, Ohnami-ike, 26 June 2010, Kenji Suetsugu Mk2 (KYO); Kirishima-shi, Makizono-cho, Ohnami-ike, 18 June 2010, Shuichi Kurogi MZ45233 (MZ); Kirishima-shi, Makizono-cho, Shinyu, 25 June 2019, Kenji Suetsugu KS427 (KYO); Tarumizu-shi, Onogaradake, 26 June 2022, Hiromitsu Sakota et al. KAG181002 (KAG). Miyazaki Pref.: Ebino-shi, Obeno, 29 June 2014, Masami Saito et al. MZ40210 (MZ); Ebino-shi, Rokkannon Mike, 18 June 2002, Shuichi Kurogi MZ45234 (MZ). Kinki District—Wakayama Pref.: Tanabe-shi, Nakahechi-cho, 28 June 2020, Tomoaki Ohe KS709 (KYO). Osaka Pref.: Izumisano-shi, Mt. Takashiro, 19 June 2021, Tetsuro Ikeda M76-1 (KYO); Kaizuka-shi, Mt. Izumi Katsuragi, 27 June 2020, Tetsuro Ikeda KS708 (KYO). Chubu District—Gifu Pref.: Ena-shi, Nakanoho-cho, 7 July 2017, Katsumi Iwahori M1 (KYO); Ena-shi, Nakanoho-cho, 18 July 2018, Katsumi Iwahori M11 (KYO); Ena-shi, Nakanoho-cho, 27 June 2020, Katsumi Iwahori KS706 (KYO); Ena-shi, Nakanoho-cho, 27 June 2020, Katsumi Iwahori KS707 (KYO). Shizuoka Pref.: Fujieda-shi, Setonoya, 8 July 2017, Masayuki Sato M10-1 (KYO); Fujieda-shi, Setonoya, 8 July 2017, Masayuki Sato M10-2 (KYO); Fujieda-shi, Setonoya, 20 June 2013, Kenji Suetsugu Mk3 (KYO); Fujieda-shi, Mt. Ryuso, 17 July 2017, Norio Nishiguchi M2 (KYO); Fujieda-shi, Mt. Ryuso, 20 June 2012, Kenji Suetsugu Mk4 (KYO).
Japanese name. Kirishima-gin-ryo-so
Etymology. The species is named after the type locality, Kirishima. To distinguish it from Monotropastrum humile f. roseum (beni-bana-gin-ryo-so, in Japanese) described by Honda (1957), we use Kirishima-gin-ryo-so as a Japanese name, after the type locality.
Distribution. Japan [Kyushu District (Kagoshima and Miyazaki), Kinki District (Wakayama and Osaka), and Chubu District (Gifu and Shizuoka)]. During intensive fieldwork and herbaria surveys, we identified several previously unknown populations of this taxon, previously considered endemic to the area around Kirishima, Kagoshima. It has now been recognized in Kyushu and Honshu. It is likely that M. kirishimense also occurs in Kochi, Shikoku, where field photographs of similar plants are shown on the website (https://hanasakiyama.web.fc2.com/yasou/sp/Itiyakusou_Benibanaginryousou.htm). Because mycoheterotrophic plants are easily overlooked in the wild because of their short flowering season and dwarf habit, M. kirishimense may be more widely distributed. In addition, M. kirishimense has probably been confused with the more well-known M. humile with similar morphology. Therefore, further surveys during the flowering season may reveal a broader distribution for M. kirishimense.
Conservation status. While we have found that Monotropastrum kirishimense is distributed in the Kyushu, Kinki, and Chubu Districts, M. kirishimense is much rarer than M. humile. The populations often harbor fewer than 20 individuals each; at the type locality, which sustains the largest number of individuals, the population comprises fewer than 50 plants. Therefore, we consider the conservation status to be endangered (EN) according to the IUCN criteria (IUCN 2019), under criterion D, in which the number of mature individuals is less than 250.
Data availability
MIG-seq and fungal community data are deposited in the DRA (DRA014598 and DRA013047, respectively).
References
Andres H (1935) Über die Pirolaceen-Gattung Monotropastrum. H Andr Notizbl Bot Gaz 12:696–699
Andres H (1936) Pirolaceae. Symbolae Sinicae: Anthophyta 7:762–768
Barrett CF, Freudenstein JV (2011) An integrative approach to delimiting species in a rare but widespread mycoheterotrophic orchid. Mol Ecol 20:2771–2786
Barrett CF, Santee MV, Fama NM et al (2022) Lineage and role in integrative taxonomy of a heterotrophic orchid complex. Mol Ecol 31:4762–5478. https://doi.org/10.1111/mec.16617
Bidartondo M, Bruns T (2001) Extreme specificity in epiparasitic Monotropoideae (Ericaceae): widespread phylogenetic and geographical structure. Mol Ecol 10:2285–2295
Bidartondo MI, Bruns TD (2002) Fine-level mycorrhizal specificity in the Monotropoideae (Ericaceae): specificity for fungal species groups. Mol Ecol 11:557–569. https://doi.org/10.1046/j.0962-1083.2001.01443.x
Bidartondo MI, Bruns TD (2005) On the origins of extreme mycorrhizal specificity in the Monotropoideae (Ericaceae): performance trade-offs during seed germination and seedling development. Mol Ecol 14:1549–1560
Buyck B, Wang XH, Adamcikova K et al (2020) One step closer to unravelling the origin of Russula: subgenus Glutinosae subg. nov. Mycosphere 11:285–305
Calcagno V, Thomas Y, Bourguet D (2007) Sympatric host races of the European corn borer: adaptation to host plants and hybrid performance: Adaptation to host plants in ECB. J Evol Biol 20:1720–1729. https://doi.org/10.1111/j.1420-9101.2007.01391.x
Chapurlat E, Roncé IL, Ågren J, Sletvold N (2020) Divergent selection on flowering phenology but not on floral morphology between two closely related orchids. Ecol Evol 10:5737–5747. https://doi.org/10.1002/ece3.6312
de Vega C, Berjano R, Arista M et al (2008) Genetic races associated with the genera and sections of host species in the holoparasitic plant Cytinus (Cytinaceae) in the Western Mediterranean basin. New Phytol 178:875–887
Drès M, Mallet J (2002) Host races in plant–feeding insects and their importance in sympatric speciation. Philos Trans R Soc B Biol Sci 357:471–492
Freudenstein JV, Barrett CF (2014) Fungal host utilization helps circumscribe leafless coralroot orchid species: an integrative analysis of Corallorhiza odontorhiza and C. wisteriana. Taxon 63:759–772
Fry JD (2003) Multilocus models of sympatric speciation: bush versus rice versus Felsenstein. Evolution 57:1735–1746
Gause GF (1934) The struggle for existence. The Williams & Wilkins company, Baltimore
Hara H (1961) New or noteworthy flowering plants from Eastern Himalaya (1). J Jpn Bot 36:75–80
Hara H (1965) New or noteworthy flowering plants from Eastern Himalaya (4). J Jpn Bot 40:97–103
Honda M (1957) Scientific names of plants of Japan. Kousei-sha, Tokyo
Hopkins R (2013) Reinforcement in plants. New Phytol 197:1095–1103. https://doi.org/10.1111/nph.12119
Hsu T-W, Kuoh C-S, Hsieh C-F (1998) Cheilotheca. In: Editorial committee of the flora of Taiwan (ed) Flora of Taiwan, 2nd ed. Department of Botany, National Taiwan University, Taipei, pp 5–6
Hynson NA, Bruns TD (2009) Evidence of a myco-heterotroph in the plant family Ericaceae that lacks mycorrhizal specificity. Proc R Soc B 276:4053–4059. https://doi.org/10.1098/rspb.2009.1190
Imamura A, Kurogi S (2003) Difference in monotropoid mycorrhiza formation between Monotropastrum globosum and its forma roseum. Mycoscience 44:63–65
Jacquemyn H, Brys R, Merckx VSFT et al (2014) Coexisting orchid species have distinct mycorrhizal communities and display strong spatial segregation. New Phytol 202:616–627. https://doi.org/10.1111/nph.12640
Jacquemyn H, Waud M, Lievens B, Brys R (2016) Differences in mycorrhizal communities between Epipactis palustris, E. helleborine and its presumed sister species E. neerlandica. Ann Bot 118:105–114. https://doi.org/10.1093/aob/mcw015
Jacquemyn H, Kort HD, Broeck AV, Brys R (2018) Immigrant and extrinsic hybrid seed inviability contribute to reproductive isolation between forest and dune ecotypes of Epipactis helleborine (Orchidaceae). Oikos 127:73–84
Keng H (1974) Rediscovery of Cheilotheca malayana and the identity of Cheilotheca, Andresia, and Monotropastrum (Ericaceae-Monotropoideae). Reinwardtia 9:77–83
Keng H, Hsieh F (1978) Cheilotheca. In: Editorial committee of the flora of Taiwan (ed) Flora of Taiwan, vol 4, Department of Botany, National Taiwan University, Taipei, p 4
Kron KA, Judd WS, Stevens PF et al (2002) Phylogenetic classification of Ericaceae: molecular and morphological evidence. Bot Rev 68:335–423
Kumar S, Stecher G, Li M et al (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Li W, Fu L, Niu B et al (2012) Ultrafast clustering algorithms for metagenomic sequence analysis. Brief Bioinform 13:656–668. https://doi.org/10.1093/bib/bbs035
Looney BP, Meidl P, Piatek MJ et al (2018) Russulaceae: a new genomic dataset to study ecosystem function and evolutionary diversification of ectomycorrhizal fungi with their tree associates. New Phytol 218:54–65
Lowry DB, Modliszewski JL, Wright KM et al (2008) The strength and genetic basis of reproductive isolating barriers in flowering plants. Philos Trans R Soc B Biol Sci 363:3009–3021. https://doi.org/10.1098/rstb.2008.0064
Matsuda Y, Okochi S, Katayama T et al (2011) Mycorrhizal fungi associated with Monotropastrum humile (Ericaceae) in central Japan. Mycorrhiza 21:569–576. https://doi.org/10.1007/s00572-011-0365-3
McCormick MK, Jacquemyn H (2014) What constrains the distribution of orchid populations? New Phytol 202:392–400
Merckx VS (2013) Mycoheterotrophy: an introduction. In: Merckx V (ed) Mycoheterotrophy: the biology of plants living on fungi. Springer, Berlin, pp 1–17
Min S, Chang-Qin Z, Yong-Peng M et al (2012) Mycorrhizal features and fungal partners of four mycoheterotrophic Monotropoideae (Ericaceae) species from Yunnan, China. Symbiosis 57:1–13
Mujic AB, Durall DM, Spatafora JW, Kennedy PG (2016) Competitive avoidance not edaphic specialization drives vertical niche partitioning among sister species of ectomycorrhizal fungi. New Phytol 209:1174–1183. https://doi.org/10.1111/nph.13677
Nilsson RH, Larsson K-H, Taylor AFS et al (2019) The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res 47:D259–D264
Osborne OG, Kafle T, Brewer T et al (2020) Sympatric speciation in mountain roses (Metrosideros) on an oceanic island. Philos Trans R Soc B Biol Sci 375:20190542. https://doi.org/10.1098/rstb.2019.0542
POWO (2022) Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. http://www.plantsoftheworldonline.org/. Accessed 6 Aug 2022
Qin HN, Wallace GD (2005) Monotropa & Monotropastrum. In: Wu ZY, Raven PH, Hong DY (eds) Flora of China, vol 14. Science Press. Beijing and Missouri Botany Garden Press, St. Louis, pp 257–259
Rochette NC, Rivera-Colón AG, Catchen JM (2019) Stacks 2: analytical methods for paired-end sequencing improve RADseq-based population genomics. Mol Ecol 28:4737–4754
Rognes T, Flouri T, Nichols B et al (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584
Rose JP, Freudenstein JV (2014) Cryptic and overlooked: species delimitation in the mycoheterotrophic Monotropsis (Ericaceae: Monotropoideae). Syst Bot 39:578–593
Rundle HD, Nosil P (2005) Ecological speciation. Ecol Lett 8:336–352. https://doi.org/10.1111/j.1461-0248.2004.00715.x
Shimono Y, Kato M, Takamatsu S (2004) Molecular phylogeny of Russulaceae (Basidiomycetes; Russulales) inferred from the nucleotide sequences of nuclear large subunit rDNA. Mycoscience 45:303–316
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Suetsugu K, Matsubayashi J (2021) Evidence for mycorrhizal cheating in Apostasia nipponica, an early-diverging member of the Orchidaceae. New Phytol 229:2302–2310
Suetsugu K, Matsubayashi J, Tayasu I (2020) Some mycoheterotrophic orchids depend on carbon from dead wood: Novel evidence from a radiocarbon approach. New Phytol 227:1519–1529. https://doi.org/10.1111/nph.16409
Suetsugu K, Haraguchi TF, Tanabe AS, Tayasu I (2021a) Specialized mycorrhizal association between a partially mycoheterotrophic orchid Oreorchis indica and a Tomentella taxon. Mycorrhiza 31:243–250
Suetsugu K, Hirota SK, Suyama Y (2021b) A new natural hybrid, Goodyera ×tanakae (Orchidaceae) from Japan with a discussion on the taxonomic identities of G. foliosa, G. sonoharae, G. velutina, G. ×maximo-velutina and G. henryi, based on morphological and molecular data. Taiwania 66:277–286
Suyama Y, Matsuki Y (2015) MIG-seq: an effective PCR-based method for genome-wide single-nucleotide polymorphism genotyping using the next-generation sequencing platform. Sci Rep 5:16963
Suyama Y, Hirota SK, Matsuo A et al (2022) Complementary combination of multiplex high-throughput DNA sequencing for molecular phylogeny. Ecol Res 37:171–181. https://doi.org/10.1111/1440-1703.12270
Tanabe AS, Toju H (2013) Two new computational methods for universal DNA barcoding: a benchmark using barcode sequences of bacteria, archaea, animals, fungi, and land plants. PLoS ONE 8:e76910. https://doi.org/10.1371/journal.pone.0076910
Taylor LD, Bruns TD (1999) Population, habitat and genetic correlates of mycorrhizal specialization in the “cheating” orchids Corallorhiza maculata and C. mertensiana. Mol Ecol 8:1719–1732. https://doi.org/10.1046/j.1365-294x.1999.00760.x
Taylor DL, Hollingsworth TN, McFarland JW et al (2014) A first comprehensive census of fungi in soil reveals both hyperdiversity and fine-scale niche partitioning. Ecol Monogr 84:3–20. https://doi.org/10.1890/12-1693.1
Thorogood CJ, Rumsey FJ, Harris SA, Hiscock SJ (2008) Host-driven divergence in the parasitic plant Orobanche minor Sm. (Orobanchaceae). Mol Ecol 17:4289–4303. https://doi.org/10.1111/j.1365-294X.2008.03915.x
Tsukaya H, Yokoyama J, Imaichi R, Ohba H (2008) Taxonomic status of Monotropastrum humile, with special reference to M. humile var. glaberrimum (Ericaceae, Monotropoideae). J Plant Res 121:271–278. https://doi.org/10.1007/s10265-008-0157-9
Wallace GD (1975) Studies of the Monotropoideae (Ericaceae): taxonomy and distribution. Wasmann J Biol 33:1–88
Wallace GD (1987) Transfer of Eremotropa sciaphila to Monotropastrum (Ericaceae, Monotropoideae). Taxon 36:128–130
Wang J, Buyck B, Wang X-H, Bau T (2019) Visiting Russula (Russulaceae, Russulales) with samples from southwestern China finds one new subsection of R. subg. Heterophyllidia with two new species. Mycol Progress 18:771–784
Waud M, Busschaert P, Ruyters S et al (2014) Impact of primer choice on characterization of orchid mycorrhizal communities using 454 pyrosequencing. Mol Ecol Resour 14:679–699. https://doi.org/10.1111/1755-0998.12229
Wu L, Jiang R-H, Yang J-C, Liu Y (2016) New records of Cheilotheca (Ericaceae, Monotropoideae) from China including the description of a new species. Phytotaxa 260:193–198
Yokoyama J, Fukuda T, Tsukaya H (2005) Molecular identification of the mycorrhizal fungi of the epiparasitic plant Monotropastrum humile var. glaberrimum (Ericaceae). J Plant Res 118:53–56. https://doi.org/10.1007/s10265-004-0188-9
Zhao Q-R, Zhou J, Peng H, Liu Z-W (2019) Resurrection of the East Asian genus Eremotropa (Monotropoideae, Ericaceae), based on molecular and morphological data. J Syst Evol 57:75–80
Acknowledgements
The authors thank Hideo Shimada, Masayuki Sato, Hisanori Takeuchi, Katsumi Iwahori, Norio Nishiguchi, Masakuni Kimura, Shin Terui, Kazushige Uemori, Tomoaki Ohe, and Tetsuro Ikeda for fieldwork assistance, or for donating samples. We thank Makoto Taniguchi, Takako Shizuka, Michiko Ishida, Hidehito Okada, and Kazuma Takizawa for technical assistance. We acknowledge the curators of KAG, KYO, MZ, TAIF, and TI for access to herbaria or collection databases. We thank Hirokazu Tsukaya, Shuichiro Tagane, Tadashi Minamitani and Masami Saito for useful discussions on species delimitation. We would like to thank Editage (www.editage.com) for English language editing. The line drawings were prepared by Kumi Hamasaki. This study was financially supported by PRESTO (JPMJPR21D6, KS) from the Japan Science and Technology Agency, and by the Environment Research and Technology Development Fund (#4-2001, KS and YS; #4-1902, YS) from the Ministry of Environment, Japan.
Author information
Authors and Affiliations
Contributions
KS planned and designed the research. KS and T-CH collected the materials and obtained the morphological data. SK and AK investigated flowering phenology. KS and HKS conducted the molecular experiments. KS, HKS, and YS carried out the molecular analyses. KS wrote the article with input from all the authors. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kenji Suetsugu is the recipient of the BSJ Award for Young Scientist, 2017.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suetsugu, K., Hirota, S.K., Hsu, TC. et al. Monotropastrum kirishimense (Ericaceae), a new mycoheterotrophic plant from Japan based on multifaceted evidence. J Plant Res 136, 3–18 (2023). https://doi.org/10.1007/s10265-022-01422-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-022-01422-8