Abstract
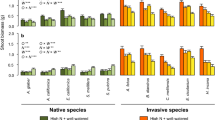
Invasive species are frequently found in recently disturbed sites. To examine how these disturbance-dependent invasive species exploit resource pulses resulting from disturbance, twelve physiological and morphological traits, including age-dependent responsiveness in leaf traits to nitrogen pulse, were compared between Bischofia javanica, an invasive tree species in Ogasawara islands, and three native Ogasawara species, each having a different successional status. When exposed to a nitrogen pulse, invasive B. javanica showed higher increases in photosynthetic capacity, leaf area, epidermal cell number and cell size in leaves of broad age classes, and root nitrogen absorption ability than two native mid-/late or late-successional species, but showed no particular superiority to a native pioneer species in these responses. Under low nitrogen, however, it showed the largest relative growth rate among the four species, while the native pioneer showed the lowest growth. From these results, we concluded that the combination of moderately high responsiveness to resource pulses and the ability to maintain steady growth under resource limitations may give B. javanica a competitive advantage over a series of native species with different successional status from early to late-successional stages.









Similar content being viewed by others
References
Alpert P, Bone E, Holzapfel C (2000) Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect Plant Ecol Evol Syst 3:52–66. doi:10.1078/1433-8319-00004
Bhuyan P, Khan M (2003) Tree diversity and population structure in undisturbed and human-impacted stands of tropical wet evergreen forest in Arunachal Pradesh, Eastern Himalayas, India. Biodivers Conserv 12:1735–1773. doi:10.1023/A:1023619017786
Burke MJ, Grime JP (1996) An experimental study of plant community invasibility. Ecology 77:776–790. doi:10.2307/2265501
Closset-Kopp D, Chabrerie O, Valentin B, Delachapelle H, Decocq G (2007) When Oskar meets Alice: does a lack of trade-off in r/K-strategies make Prunus serotina a successful invader of European forests? Forest Ecol Manag 247:120–130. doi:10.1016/j.foreco.2007.04.023
Coley PD, Kursar T (1996) Anti-herbivore defenses of young tropical leaves: physiological constraints and ecological trade-offs: In: Mulkey SS, Chazdon RL, Smith AP (eds) Tropical Forest Plant Ecophysiology. Springer, New York, pp 305–336
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Ann Rev Ecol Evol Syst 34:183–211
D’Antonio CM, Dudley TL, Mack M (1999) Disturbance and biological invasions: Direct effects and feedbacks. In: Walker LR (ed) Ecosystems of disturbed ground. Elsevier, New York, pp 413–452
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol Lett 14:419–431. doi:10.1111/j.1461-0248.2011.01596.x
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534. doi:10.1046/j.1365-2745.2000.00473.x
Denslow JS, Space JC (2009) Invasive exotic plants in the tropical Pacific Islands: patterns of diversity. Biotropica 41:162–170. doi:10.1111/j.1744-7429.2008.00469.x
Denslow JS, Ellison AM, Sanford RE (1998) Treefall gap size effects on above- and below- ground processes in a tropical wet forest. J Ecol 86:597–609. doi:10.1046/j.1365-2745.1998.00295.x
Drown DM, Levri EP, Dybdahl MF (2011) Invasive genotypes are opportunistic specialists not general purpose genotypes. Evol Appl 4:132–143. doi:10.1111/j.1752-4571.2010.00149.x
Fricke W, Mcdonald AJS, Mattson-Djos L (1997) Why do leaves and leaf cells of N-limited barley elongate at reduced rates? Planta 202:522–530. doi:10.1007/s004250050157
Funk JL (2008) Differences in plasticity between invasive and native plants from a low resource environment. J Ecol 96:1162–1173. doi:10.1111/j.1365-2745.2008.01435.x
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081. doi:10.1038/nature05719
Gelman A, Carlin JB, Stern HS, Rubin DB (2004) Bayesian data analysis Chapman & Hall/CRC, London
Gibson N, Keighery G, Keighery B (2000) Threatened plant communities of the western Australia 1, the ironstone communities of the Swan and Scott Coastal Plains. J R Soc Western Aust 83:1–11
Godoy O, Valladares F, Castro-Díez P (2011) Multispecies comparison reveals that invasive and native plants differ in their traits but not in their plasticity. Funct Ecol 25:1248–1259. doi:10.1111/j.1365-2435.2011.01886.x
Godoy O, Valladares F, Castro-Díez P (2012) The relative importance for plant invasiveness of trait means, and their plasticity and integration in a multivariate framework. New Phytol 195:912–922. doi:10.1111/j.1469-8137.2012.04205.x
Granier C, Tardieu F (2009) Multi-scale phenotyping of leaf expansion in response to environmental changes: the whole is more than the sum of parts. Plant Cell Env 32:1175–1184. doi: 10.1111/j.1365-3040.2009.01955.x
Hanba YT, Kogami H, Terashima I (2002) The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand. Plant Cell Env 25:1021–1030
Hata K, Suzuki JI, Kachi N, Yamamura Y (2006) A 19-year study of the dynamics of an Invasive Alien Tree, Bischofia javanica, on a Subtropical Oceanic Island 1. Pac Sci 60:455–470. doi:10.1353/psc 2006.0029
Hierro JL, Maron JL, Callaway RM (2005) A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. J Ecol 93:5–15. doi:10.1111/j.0022-0477.2004.00953.x
Hunt R (1982) Plant growth analysis. The Lavenham Press Limited, Suffolk
Jurik TW, Chabot JF, Chabot BF (1979) Ontogeny of photosynthetic performance in Fragaria virginiana under changing light regimes. Plant Physiol 63:542–547. doi:10.1104/pp.63.3.542
Kamaluddin M, Grace J (1992) Photoinhibition and light acclimation in seedlings of Bischofia javanica, a tropical forest tree from Asia. Annal Bot 69:47–52
Kamo K (2003) Bichofia javanica in Mt Kinabalu, Malaysia. Trop For 58:17–24 (in Japanese)
Kitayama K, Mueller-Dombois D (1995) Biological invasion on an oceanic island mountain: Do alien plant species have wider ecological ranges than native species? J Veg Sci 6:667–674. doi:10.2307/3236436
Kobayashi S (1978) A list of the vascular plants occuring in the Ogasawara (Bonin) Islands. Ogasawara Res 1:1–33
Kueffer C, Kronauer L, Edwards PJ (2009) Wider spectrum of fruit traits in invasive than native floras may increase the vulnerability of oceanic islands to plant invasions. Oikos 118:1327–1334. doi:10.1111/j.1600-0706.2009.17185.x
Leishman MR, Thomson VP (2005) Experimental evidence for the effects of additional water, nutrients and physical disturbance on invasive plants in low fertility Hawkesbury Sandstone soils, Sydney, Australia. J Ecol 93:38–49. doi:10.1111/j.1365-2745.2004.00938.x
Lemmens RHMJ, Soerianegara I, Wong WC (1995) Timber trees: minor commercial timbers: plant resources of south-east Asia. Backhuys Publishers, Leiden, pp 324–325
Naidu SL, DeLucia EH (1997) Growth, allocation and water relations of shade-grown Quercus rubra L. saplings exposed to a late-season canopy gap. Annal Bot 80:335–344. doi:10.1023/A:1009780114992
Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light-acclimation need change in leaf anatomy?. Plant Cell Env 26:505–512
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Env 28:916–927. doi:10.1111/j.1365-3040.2005.01344.x->
Oguchi R, Hikosaka K, Hiura T, Hirose T (2006) Leaf anatomy and light acclimation in woody seedlings after gap formation in a cool-temperate deciduous forest. Oecologia 149:571–582. doi:10.1007/s00442-006-0485-1
Ono K, Watanabe A (1997) Levels of endogenous sugars, transcripts of rbcS and rbcL, and of RuBisCO protein in senescing sunflower leaves. Plant Cell Physiol 38:1032–1038
Palacio-López K, Gianoli E (2011) Invasive plants do not display greater phenotypic plasticity than their native or non-invasive counterparts: a meta-analysis. Oikos 120:1393–1401. doi:10.1111/j.1600-0706.2010.19114.x
Paquette A, Fontaine B, Berninger F, Dubois K, Lechowicz MJ, Messier C, Posada JM, Valladares F, Brisson J (2012) Norway maple displays greater seasonal growth and phenotypic plasticity to light than native sugar maple. Tree Physiol 32:1339–1347. doi:10.1093/treephys/tps092
Parsons WFJ, Knight DH, Miller SL (1994) Root gap dynamics in lodgepole pine forest: nitrogen transformations in gaps of different size. Ecol Appl 4:354–362. doi:10.2307/1941939
Pattison RR, Goldstein G, Ares A (1998) Growth, biomass allocation and photosynthesis of invasive and native Hawaiian rainforest species. Oecologia 117:449–459. doi:10.1007/s004420050680
Pearcy RW, Sims DA (1994) Photosynthetic acclimation to changing light environments: scaling from the leaf to the whole plant. In: Caldwell MM, Pearcy RW (eds) Exploitation of environmental heterogeneity by plants: ecophysiological processes above and below ground. Academic Press, San Diego, pp 145–174
Pigliucci M (2005) Evolution of phenotypic plasticity: where are we going now? Trends Ecol Evol 20:481–486. doi:10.1111/j.1365-2745.2008.01435.x
Poorter L (1999) Growth responses of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Funct Ecol 13:396–410. doi:10.1046/j.1365-2435.1999.00332.x
Poorter L, van de Plassche M, Willems S, Boot RGA (2004) Leaf traits and herbivory rates of tropical tree species differing in successional status. Plant Biol 6:746–754. doi:10.1055/s-2004-821269
Reich PB, Tjoelker MG, Walters MB, Vanderklein DW, Buschena C (1998) Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light. Funct Ecol 12:327–338. doi:10.1046/j.1365-2435.1998.00208.x
Reich PB, Ellsworth D, Walters M, Vose J, Gresham C, Volin J, Bowman W (1999) Generality of leaf trait relationships: a test across six biomes. Ecology 80:1955–1969. doi:10.1890/0012-9658(1999)080
Rejmánek M (1989) Invasibility of plant communities. In: Drake JA, Mooney HA, di Castri F, Groves RH, Kruger FJ, Rejmanek M, Williamson M (eds) Biological invasions: a global perspective. John Wiley, New York, pp 369–388
Rejmánek M, Richardson DM, Pysek P (2005) Plant invasions and invasibility of plant communities, In: van der Maarel (ed) Vegetation ecology. Blackwell Publishing, Oxford, pp 332–355
Roggatz U, Donald AJSMC, Stadenberg I, Schurr U (1999) Effects of nitrogen deprivation on cell division and expansion in leaves of Ricinus communis L. Plant Cell Env 22:81–89. doi:10.1046/j.1365-3040.1999.00383.x
Sakai AK, Wagner WL, Mehrhoff LA (2002) Patterns of endangerment in the Hawaiian flora. Syst Biol 51:276–302. doi:10.1080/10635150252899770
Sanford NL, Harrington RA, Fownes JH (2003) Survival and growth of native and alien woody seedlings in open and understory environments. For Ecol Manag 183:377–385. doi:10.1016/S0378-1127(03)00141-5
Scharenbroch BC, Bockheim JG (2007) Impacts of forest gaps on soil properties and processes in old growth northern hardwood-hemlock forests. Plant Soil 294:219–233. doi:10.1007/s11104-007-9248-y
Schlichting CD (1986) The evolution of phenotypic plasticity in plants. Annu Rev Ecol Syst 17:667–693
Schumacher E, Kueffer C, Edwards PJ, Dietz H (2008) Influence of drought and shade on seedling growth of native and invasive trees in the Seychelles. Biotropica 40:543–549. doi:10.1111/j.1744-7429.2008.00407.x
Schumacher E, Kueffer C, Edwards PJ, Dietz H (2009) Influence of light and nutrient conditions on seedling growth of native and invasive trees in the Seychelles. Biol Invasions 11:1941–1954. doi:10.1007/s10530-008-9371-6
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176. doi:10.1016/S0169-5347(02)02495-3
Shimizu Y (1988) Vegetation of Mt. Kuwanoki in the Bonin (Ogasawara) Islands with reference to the invasion of an introduced tree species Bischofia javanica. Regional Views 1:31–46 (in Japanese)
Shimizu Y, Tabata H (1991) Forest structure, composition and distribution on a Pacific island with reference to ecological release and speciation. Pac Sci 45:28–49
Sims DA, Pearcy RW (1992) Response of leaf anatomy and photosynthetic capacity in Alocasia macrorrhiza (Araceae) to a transfer from low to high light. Am J Bot 79:449–455
Sugiyama S (2005) Developmental basis of interspecific differences in leaf size and specific leaf area among C3 grass species. Funct Ecol 19(916):924. doi:10.1111/j.1365-2435.2005.01044.x
Sultan SE (2001) Phenotypic plasticity for fitness components in Polygonum species of contrasting ecological breadth. Ecology 82:28–343. doi:10.1111/j.1399-3054.1982.tb00282.x
Tanimoto T, Toyoda T (1996) Survivorship and growth of Akagi (Bischofia javanica BI) seedlings under the forest canopy and different temperature conditions. Bull For For Prod Res Inst 370:1–19 (in Japanese with English summary)
Tilman D (2004) Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci USA 101:10854–10861. doi:10.1073/pnas.0403458101
Toyoda T. (2003) Flora of Bonin Islands (Enlarged & Revised). Aboc, Kanagawa
Trapani N, Hall AJ, Weber M (1999) Effects of constant and variable nitrogen supply on sunflower (Helianthus annuus L.) leaf cell number and size. Ann Bot 84:599–606. doi:10.1006/anbo 1999.0954
Turnbull MH, Doley D, Yates DJ (1993) The dynamics of photosynthetic acclimation to changes in light quantity and quality in three Australian rainforest tree species. Oecologia 94:218–228. doi:10.1007/BF00341320
van Staden J, Carmi A (1982) The effects of decapitation on the distribution of cytokinins and growth of Phaseolus vulgaris plants. Physiol Plantarum 55:39–44. doi:10.1111/j.1399-3054.1982.tb00282.x
Yamashita N, Koike N, Ishida A (2002) Leaf ontogenetic dependence of light acclimation in invasive and native subtropical trees of different successional status. Plant Cell Env 25:1341–1356. doi:10.1046/j.1365-3040.2002.00907.x
Yamashita N, Tanaka N, Hoshi Y, Kushima H, Kamo K (2003) Seed and seedling demography of invasive and native trees of subtropical Pacific islands. J Veg Sci 14:15–24. doi:10.1111/j.1654-1103.2003.tb02123.x
Acknowledgments
We thank Naoko Yamashita and Shuichi Sugiyama for helpful advice in the analysis and collection of data, and Kouki Hikosaka for valuable comments of earlier version of this manuscript. Two anonymous reviewers provided insightful comments on the manuscript. This work was supported by Grant-in-aid of the Japan Ministry of Education, Science and Culture [18770022, 24370009] and by grant-in-aid from Fujiwara Natural History foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osone, Y., Yazaki, K., Masaki, T. et al. Responses to nitrogen pulses and growth under low nitrogen availability in invasive and native tree species with differing successional status. J Plant Res 127, 315–328 (2014). https://doi.org/10.1007/s10265-013-0609-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-013-0609-8