Abstract
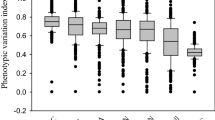
Although broad-scale inter-specific patterns of leaf traits are influenced by climate, soil, and taxonomic identity, integrated assessments of these drivers remain rare. Here, we quantify these drivers in a field study of 171 plant species in 174 sites across Chinese grasslands, including the Tibetan Plateau, Inner Mongolia, and Xinjiang. General linear models were used to partition leaf trait variation. Of the total variation in leaf traits, on average 27% is due to taxonomic or phylogenetic differences among species within sites (pure species effect), 29% to variation among sites within species (pure site effect), 38% to joint effects of taxonomic and environmental factors (shared effect), and 6.2% to within-site and within-species variation. Examining the pure site effect, climate explained 7.8%, soil explained 7.4%, and climate and soil variables together accounted for 11%, leaving 18% of the inter-site variation due to factors other than climate or soil. The results do not support the hypothesis that soil fertility is the “missing link” to explain leaf trait variation unexplained by climatic factors. Climate- and soil-induced leaf adaptations occur mostly among species, and leaf traits vary little within species in Chinese grassland plants, despite strongly varying climate and soil conditions.


Similar content being viewed by others
References
Aerts R, Chapin FS III (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Angiosperm Phylogeny Group (2003) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot J Linn Soc 141:399–436
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73:1045–1055
Bowman RA (1988) A rapid method to determine total phosphorus in soils. Soil Sci Soc Am J 52:1580–1585
Campbell GS, Norman JM (1998) An introduction to environmental biophysics. Springer, New York
Castro-Díez P, Puyravaud JP, Cornelissen JHC (2000) Leaf structure and anatomy as related to leaf mass per area variation in seedlings of a wide range of woody plant species and types. Oecologia 124:476–486
Chapin FS III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Craine JM, Lee WG, Bond WJ, Williams RJ, Johnson LC (2005) Environmental constraints on a global relationship among leaf and root traits of grasses. Ecology 86:12–19
Crisp MD, Arroyo MTK, Cook LG, Gandolfo MA, Jordan GJ, McGlone MS, Weston PH, Westoby M, Wilf P, Linder HP (2009) Phylogenetic biome conservatism on a global scale. Nature 458:754–756
Díaz S, Lavorel S, McIntyre S, Falczuk V, Casanoves F, Milchunas DG, Skarpe C, Rusch G, Sternberg M, Noy-Meir I, Landsberg J, Zhang W, Clark H, Campbell BD (2007) Plant trait responses to grazing—a global synthesis. Glob Change Biol 13:313–341
Garnier E, Salager J-L, Laurent GLS (1999) Relationships between photosynthesis, nitrogen and leaf structure in 14 grass species and their dependence on the basis of expression. New Phytol 143:119–129
Garnier E, Lavorel S, Ansquer P, Castro H, Cruz P, Dolezal J, Eriksson O, Fortunel C, Freitas H, Golodets C, Grigulis K, Jouany C, Kazakou E, Kigel J, Kleyer M, Lehsten V, Lepš J, Meier T, Pakeman R, Papadimitriou M, Papanastasis VP, Quested H, Quétier F, Robson M, Roumet C, Rusch G, Skarpe C, Sternberg M, Theau JP, Thébault A, Vile D, Zarovali MP (2007) Assessing the effects of land-use change on plant traits, communities and ecosystem functioning in grasslands: a standardized methodology and lessons from an application to 11 European sites. Ann Bot 99:967–985
Grime JP (1979) Plant strategies and vegetation processes. Wiley, Chichester
He J-S, Fang JY, Wang ZH, Guo D, Flynn DFB, Geng Z (2006a) Stoichiometry and large-scale patterns of leaf carbon and nitrogen in the grassland biomes of China. Oecologia 149:115–122
He J-S, Wang ZH, Wang XP, Schmid B, Zuo WY, Zhou M, Zheng CY, Wang M, Fang JY (2006b) A test of the generality of leaf trait relationships on the Tibetan Plateau. New Phytol 170:835–848
He J-S, Wang L, Flynn DFB, Wang X, Ma W, Fang J (2008) Leaf nitrogen:phosphorus stoichiometry across Chinese grassland biomes. Oecologia 155:301–310
He J-S, Wang XP, Flynn DFB, Wang L, Schmid B, Fang JY (2009) Taxonomic, phylogenetic and environmental trade-offs between leaf productivity and persistence. Ecology 90:2779–2791
Hikosaka K (2004) Interspecific difference in the photosynthesis-nitrogen relationship: patterns, physiological causes, and ecological importance. J Plant Res 117:481–494
Hirose T, Werger MJA (1994) Photosynthetic capacity and nitrogen partitioning among species in the canopy of a herbaceous plant community. Oecologia 100:203–212
Hooper DU, Johnson L (1999) Nitrogen limitation in dryland ecosystems: responses to geographical and temporal variation in precipitation. Biogeochemistry 46:247–293
Institute SAS (1999) SAS/STAT user’s guide, Version 8.01 (http://support.sas.com). SAS Institute, Cary
Jenny H (1941) Factors of soil formation. McGraw-Hill, New York
Kuo S (1996) Phosphorus. In: Bigham JM (ed) Methods of soil analysis. Part 3, chemical methods. Soil Science Society of America, American Society of Agronomy, Madison, WI, pp 869–919
Lavorel S, Díaz S, Cornelissen JHC, Garnier E, Harrison SP, McIntyre S, Pausas JG, Pérez-Harguindeguy N, Roumet C, Urcelay C (2007) Plant functional types: are we getting any closer to the Holy Grail? In: Canadell J, Pitelka LF, Pataki D (eds) Terrestrial ecosystems in a changing world. IGBP book series. Springer, Heidelberg, pp 149–160
Luo T, Luo J, Pan Y (2005) Leaf traits and associated ecosystem characteristics across subtropical and timberline forests in the Gongga Mountains, Eastern Tibetan Plateau. Oecologia 142:261–273
McGroddy ME, Daufresne T, Hedin LO (2004) Scaling of C:N:P stoichiometry in forests worldwide: implications of terrestrial Redfield-type ratios. Ecology 85:2390–2401
Niinemets Ü (2001) Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82:453–469
Niinemets Ü, Valladares F (2006) Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol Monogr 76:521–547
Niinemets Ü, Kull O, Tenhunen JD (1999) Variability in leaf morphology and chemical composition as a function of canopy light environment in coexisting deciduous trees. Int J Plant Sci 160:837–848
Ordoñez JC, van Bodegom PM, Witte J-PM, Wright IJ, Reich PB, Aerts R (2009) A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob Ecol Biogeogr 18:137–149
Piao SL, Fang JY, He JS (2006) Variations in vegetation net primary production in the Qinghai-Xizang Plateau, China, from 1982 to 1999. Clim Change 74:253–267
R Development Core Team (2007) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Nat Acad Sci 101:11001–11006
Reich PB, Uhl C, Walters MB, Ellsworth DS (1991) Leaf lifespan as a determinant of leaf structure and function among 23 Amazonian tree species. Oecologia 86:16–24
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734
Reich PB, Wright IJ, Cavender-Bares J, Craine JM, Oleksyn J, Westoby M, Walters MB (2003) The evolution of plant functional variation: traits, spectra, and strategies. Int J Plant Sci 164:S143–S164
Schlesinger WH (1997) Biogeochemistry: an analysis of global change. Academic, San Diego
Schmid B, Bazzaz FA (1994) Crown construction, leaf dynamics, and carbon gain in two perennials with contrasting architecture. Ecol Monogr 64:177–203
Schmid B, Hector A, Huston MA, Inchausti P, Nijs I, Leadley PW, Tilman D (2002) The design and analysis of biodiversity experiments. In: Loreau M, Naeem S, Inchausti P (eds) Biodiversity and ecosystem functioning: synthesis and perspectives. Oxford University Press, Oxford, pp 61–75
Shipley B, Lechowicz MJ (2000) The functional co-ordination of leaf morphology, nitrogen concentration, and gas exchange in 40 wetland species. Ecoscience 7:183–194
Slatkin M (1980) Ecological character displacement. Ecology 61:163–177
Stevens PF (2008) Angiosperm phylogeny website. http://www.mobot.org/MOBOT/research/APweb/. Version 8, June 2007
Thornthwaite CW (1948) An approach toward a rational classification of climate. Geogr Rev 38:57–94
Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D (2005) Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol 167:493–508
Townsend AR, Cleveland CC, Asner GP, Bustamante MMC (2007) Controls over foliar N:P ratios in tropical rain forests. Ecology 88:107–118
Walter H (1970) Vegetationszonen und Klima. Ulmer, Stuttgart
Webb C, Ackerly D, Kembel SW (2008) Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24(18):2098–2100
Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Syst 33:125–159
Whittaker RH (1975) Communities and ecosystems. MacMillan, New York
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee WJ, Lusk C, Midgley JJ, Navas M-L, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, Lamont BB, Lee W, Oleksyn J, Osada N, Poorter H, Villar R, Warton DI, Westoby M (2005a) Assessing the generality of global leaf trait relationships. New Phytol 166:485–496
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Groom PK, Hikosaka K, Lee W, Lusk CH, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Warton DI, Westoby M (2005b) Modulation of leaf economic traits and trait relationships by climate. Glob Ecol Biogeogr 14:411–421
Wright IJ, Reich PB, Atkin OK, Lusk CH, Tjoelker MG, Westoby M (2006) Irradiance, temperature and rainfall influence leaf dark respiration in woody plants: evidence from comparisons across 20 sites. New Phytol 169:309–319
Yang YH, Fang JY, Tang YH, Ji CJ, Zheng CY, He JS, Zhu B (2008) Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Glob Change Biol 14:1592–1599
Acknowledgments
The authors are grateful to Cunzhu Liang, Zhongling Liu, Zongyuan Zhu, and Qing Du for plant species identification in the field, and Shilong Piao for providing climate data. This research was supported by the National Natural Science Foundation of China (Grant 30870381 to J.-S.H. and A3 Foresight Program to J.F.) and the Ministry of Science and Technology of People’s Republic of China (Project 2007BAC06B01 to J.-S.H.). J.-S.H. was supported partially by a Sino-Swiss Science and Technology Cooperation Research Fellowship Program of the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, JS., Wang, X., Schmid, B. et al. Taxonomic identity, phylogeny, climate and soil fertility as drivers of leaf traits across Chinese grassland biomes. J Plant Res 123, 551–561 (2010). https://doi.org/10.1007/s10265-009-0294-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-009-0294-9