Abstract
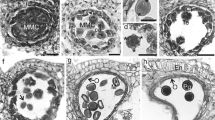
We describe details of anatomically preserved fossil glossopterid ovules from the Late Permian of Queensland, Australia, that contain several pollen tubes at various stages of releasing flagellated sperm. Each sperm is approximately 12.7 μm long and 13.9 μm wide, with a conspicuous spiral structure comprised of a series of dots that resemble the position of basal bodies of flagella aligned along the multilayered structure (MLS). This configuration is similar to the helically arranged flagella in the sperm of cycads, Ginkgo, and many pteridophytes. However, the motile gametes of Glossopteris are considerably smaller than those of Ginkgo and cycads, and more similar in size, number of basal bodies, and number of gyres in their helix to pteridophyte forms. Glossopteris thus shares the intermediate stage of motile male gamete formation and apparently that of haustorial pollen tubes with cycads and Ginkgo.


Similar content being viewed by others
References
Basinger JF, Rothwell GW (1977) Anatomically preserved plants from the Middle Eocene (Allenby Formation) of British Columbia. Can J Bot 55:1984–1990
Benson M (1908) On the contents of the pollen chamber of a specimen of Lagenostoma ovoides. Bot Gaz 45:409–442
Bierhorst, DW (1971) Morphology of vascular plants. Macmillan, New York
Bold HC, La Claire JW II (1985) The plant kingdom, 5th edn. Prentice Hall, Englewood Cliffs
Caldwell O (1907) Mycrocycas calocoma. Bot Gaz 44:118–141
Chamberlain CJ (1909) Spermatogenesis in Dioon edule. Bot Gaz 47:215–236
Choi JS, Friedman WE (1991) Development of pollen tube of Zamia furfuracea (Zamiaceae) and its evolutionary implications. Am J Bot 78:544–560
Cooper GM (2000) The cell: a molecular approach, 2nd edn. ASM Press, Washington, DC
Doyle J (1988) Pollen evolution in seed plants: a cladistic perspective. J Palynol 23–24:7–18
Doyle J, Donoghue MJ (1986) Seed plant phylogeny and the origin of angiosperms: an experimental cladistic approach. Bot Rev 52:331–429
Friedman WE (1987) Growth and development of male gametophyte of Ginkgo biloba within the ovule (in vivo). Am J Bot 74:1797–1815
Friedman WE (1993) The evolutionary history of the seed plant male gametophyte. Trends Ecol Evol 8:15–21
Friedman WE, Gifford EM (1997) Development of the male gametophyte of Ginkgo biloba: a window into the reproductive biology of early seed plants. In: Hori T, Ridge RW, Tulecke W, Del Tredici P, Tremouillaux-Guiller J, Tobe H (eds) Ginkgo biloba: a global treasure. Springer, Berlin Heidelberg New York, pp 29–49
Gifford EM, Foster AS (1989) Morphology and evolution of vascular plants, 3rd edn. Freeman, New York
Gould RE, Delevoryas T (1977) The biology of Glossopteris: evidence from petrified seed-bearing and pollen-bearing organs. Alcheringa 1:387–399
Hirase S (1896) On the spermazoid of Ginkgo biloba. Bot Mag Tokyo 10:325–328
Hori T, Miyamura S (1997) Contribution to the knowledge of fertilization of gymnosperms with flagellated sperm cells: Ginkgo biloba and Cycas revoluta. In: Hori T, Ridge RW, Tulecke W, Del Tredici P, Tremouillaux-Guiller J, Tobe H (eds) Ginkgo biloba: a global treasure. Springer, Berlin Heidelberg New York, pp 67–84
Ikeno S (1896) Spermatozoid of Cycas revoluta. Bot Mag Tokyo 10: 367–368 (in Japanese)
Ikeno S (1898) Untersuchungen über die Entwickelung der Geschlechtsorgane und den Vorgang der Befruchtung bei Cycas revoluta. Jahrb Wiss Bot 32:557–602, pls 8–10
Lee CL (1955) Fertilization in Ginkgo biloba. Bot Gaz 117:79–100
Li Y, Wang FH, Knox RB (1989) Ultrastructural analysis of the flagellar apparatus in sperm cells of Ginkgo biloba. Protoplasma 149:57–63
Lindström S, McLoughlin S, Drinnan AN (1997) Intraspecific variation of taeniate biscaccate pollen within Permian glossopterid sporangia, from the Prince Charles Mountains, Antarctica. Int J Plant Sci 158:673–684
Manton I (1959) Observations on the microanatomy of the spermatozoid of the bracken fern (Pteridium aquilinum). J Biochem Cytol 6:413–418
McManus HA, Taylor EA, Taylor TN, Collinson JW (2002) A petrified Glossopteris flora from Collinson Ridge, central Transarctic Mountains: Late Permian or Early Triassic? Rev Palaeobot Palynol 120:233–246
Melville R (1983) Glossopteridae, Angiospermidae and the evidence for angiosperm origin. Bot J Linn Soc 86:279–323
Mizukami I, Gall J (1966) Centriole replication. II. Sperm formation in the fern, Marsilea, and the cycad, Zamia. J Cell Biol 29:97–111
Nishida H, Pigg KB, Rigby JF (2003) Swimming sperm in an extinct Gondwanan plant. Nature 422:396–397
Norstog KJ (1986) The blepharoplast of Zamia pumila L. Bot Gaz 147:40–46
Pant DD (1977) The plant of Glossopteris. J Indian Bot Soc 56:1–23
Pant DD, Singh RS (1974) On the stem and attachment of Glossopteris and Gangamopteris leaves Part II—structural features. Palaeontographica 147B: 42–73, 8 pls
Pigg KB, McLoughlin S (1997) Anatomically preserved Glossopteris leaves from the Bowen and Sydney basins, Australia. Rev Palaeobot Palynol 97:339–359
Pigg KB, Taylor TN (1989) Permineralized Glossopteris and Dicroidium in Antarctica. In: Taylor TN, Taylor EL (eds) Antarctic paleobiology. Springer, Berlin Heidelberg New York, pp 164–172
Pigg KB, Trivett ML (1994) Evolution of the glossopterid gymnosperms from Permian Gondwana. J Plant Res 107:461–477
Renzagalia KS, Garbary DJ (2001) Motile gametes of land plants: diversity, development, and evolution. CRC Crit Rev Plant Sci 20:107–213
Retallack G, Dilcher DL (1988) Arguments for a glossopterid ancestry of angiosperms. Paleobiology 7:54–67
Rothwell GW (1972) Evidence of pollen tubes in Paleozoic pteridosperms. Science 175:772–774
Rothwell GW, Serbet R (1994) Lignophyte phylogeny and the evolution of spermatophytes: a numerical cladistic analysis. Syst Bot 19:443–482
Sedgwick PJ (1924) The life-history of Encephalartos. Bot Gaz 77:300–310, 2 pls
Stewart WN (1951) A new Pachytesta from the Berryville locality of Southeastern Illinois. Am Midl Natur 46:717–742
Stewart WN, Rothwell GW (1993) Paleobotany and the evolution of plants, 2nd edn. Cambridge University Press, Cambridge
Stockey RA, Rothwell, GW (2003) Anatomically preserved Williamsonia (Williamsoniaceae): evidence for Bennettitalean reproduction in the Late Cretaceous of western North America. Int J Plant Sci 164:251–262
Surange KR, Chandra S (1978) Morphology and affinities of Glossopteris. Palaeobotanist 26:509–524
Taylor EL, Taylor TN (1992) Reproductive biology of the Permian Glossopteridales and their suggested relationship to flowering plants. Proc Natl Acad Sci USA 89:11495–11497
Webber HJ (1897) The development of the antherozoids of Zamia. Bot Gaz 24:16–22
Zavada MS (1991) The ultrastructure of pollen found in the dispersed sporangia of Arberiella (Glossopteridaceae). Bot Gaz 152:248–255
Acknowledgments
We thank Mr. Hiromichi Yano for preparing the microscopic slides. The field collection at Homevale was supported by Grants for Overseas Survey from the Ministry of Education, Culture, Sports, Science and Technology No. 04041034 to Prof. Masahiro Kato, University of Tokyo, and No. 08041135 to Dr. Motomi Ito, University of Tokyo, to whom we are deeply grateful. The work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology No. 07640933 to H.N., and NSF grant BSR-9006625 and an Arizona State University Faculty Grant-in-Aid to K.B.P.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10265-004-0176-0
Rights and permissions
About this article
Cite this article
Nishida, H., Pigg, K.B., Kudo, K. et al. Zooidogamy in the Late Permian genus Glossopteris. J Plant Res 117, 323–328 (2004). https://doi.org/10.1007/s10265-004-0164-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-004-0164-4