Abstract
Advanced diffuse large B cell lymphoma (DLBCL) is a common malignant tumor with aggressive clinical features and poor prognosis. At present, there is lack of effective prognostic tool for patients with advanced (stage III/IV) DLBCL. The aim of this study is to identify prognostic indicators that affect survival and response and establish the first survival prediction nomogram for advanced DLBCL. A total of 402 patients with advanced DLBCL were enrolled in this study. COX multivariate analysis was used to obtain independent prognostic factors. The independent prognostic factors were included in the nomogram, and the nomogram to predict the performance of the model was established by R rms package, C-index (consistency index), AUC curve and calibration curve. The training and validation cohorts included 281 and 121 patients. In the training cohort, multivariate analysis showed that Ki-67 (70% (high expression) vs ≤ 70% (low expression), p < 0.001), LDH (lactate dehydrogenase) (elevated vs normal, p = 0.05), FER (ferritin) (elevated vs normal, p < 0.001), and β2-microglobulin (elevated vs normal, p < 0.001) were independent predictors and the nomogram was constructed. The nomogram showed that there was a significant difference in OS among the low-risk, intermediate-risk and high-risk groups, with 5-year survival rates of 81.6%, 44% and 6%, respectively. The C-index of the nomogram in the training group was 0.76. The internal validation of the training group showed good consistency. In the internal validation cohort of the training group, the AUC was 0.828, and similar results were obtained in the validation group, with a C-index of 0.74 and an AUC of 0.803. The proposed nomogram provided a valuable individualized risk assessment of OS in advanced DLBCL patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DLBCL is the most common type of non-Hodgkin's lymphoma, accounting for about 30–40% of all non-Hodgkin's lymphomas [1], with strong heterogeneity and poor prognosis [2, 3]. According to the Ann Arbor staging system, about 60–70% of DLBCL patients have stage III/IV at the time of initial diagnosis and advanced stage is important factor that indicating poor prognosis [4, 5]. The 10-year overall survival (OS) rate of advanced DLBCL is typically 43% [6]. Although 50–60% of DLBCL patients can be cured by the current first-line R-CHOP(rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone) regimen [7,8,9], about 40% of patients are still at risk of disease recurrence [10,11,12] and the prognosis of patients with advanced DLBCL is still poor [13]. For patients with advanced DLBCL, the first-line treatment is usually R-CHOP or R-DA-EPOCH (Rituximab, etoposide, vindesine, epirubicin, cyclophosphamide, prednisone) [14] chemotherapy [5, 15]. For initial large tumors (>7.5 cm) is usually followed by radiotherapy [13, 16]. In addition, patients with advanced DLBCL are also more likely to experience treatment failure than those with early-stage DLBCL [17]. Approximately 90% of early-stage DLBCL patients achieved long-term disease control with R-CHOP, compared with only 60% of advanced DLBCL patients [5].
Since the 1990s, the IPI score constructed from disease stage, age, serum lactate dehydrogenase (LDH) concentration, Eastern Cooperative Oncology Group (ECOG), performance status (PS), and the number of extranodal sites has been a standard tool for risk stratification and treatment guidance [18]. In the IPI score, stage is an important prognostic factor, and the higher the stage, the worse the prognosis [19]. However, there is no prognostic tool for advanced-stage DLBCL, and the means to guide precise treatment are limited. Therefore, advanced-stage DLBCL urgently needs a good prognostic tool to guide treatment.
Unlike traditional models such as IPI and R-IPI, the nomogram is a visualized statistical predictive model that identifies points of value for each variable [20], thereby improving the predictive accuracy of clinical outcomes. Previously, nomogram was demonstrated in DLBCL as well as in several malignancies, showing accurate estimates of patient survival [21, 22]. The aim of this study is to evaluate the clinical characteristics, identify the prognostic indicators that affect the survival and efficacy, and establish the first survival prediction nomogram for advanced DLBCL, which is compared with the traditional IPI prognostic system.
Patients and methods
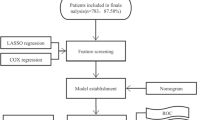
Patients with advanced DLBCL who were initially treated in 12 hospitals (Sichuan Province Cancer Hospital, Sun Yat-sen University Affiliated Cancer Hospital, Hunan Province Cancer Hospital, Guizhou Province Cancer Hospital, Fujian Province Cancer Hospital, Jiangxi Province Cancer Hospital, Yunnan Province Cancer Hospital, Liuzhou People's Hospital, the First People's Hospital of Yunnan Province, Mianyang Central Hospital, the Sixth Affiliated Hospital of Sun Yat-sen University, Henan Province Cancer Hospital.) from February 2012 to November 2022 were collected. The inclusion criteria for this study were as follows: Patients with histologically confirmed DLBCL, the Ann Arbor stage of lymphoma was stage III/IV (advanced), and complete clinical data were enrolled. Staging procedures include medical history, physical examination, hematology (including blood routine, biochemical routine, blood calcium, LDH, etc.), neck, chest, abdomen, pelvis enhanced CT or whole body PET-CT, bone marrow puncture smear and biopsy, and add gastroenteroscopy to those considered to have gastrointestinal band invasion. Ann Arbor staging was followed: invasion of a single lymph node region (I) or a single external node (I E). Invasion of more than 2 lymph node regions, but both in the same side of the diaphragm (II), may be accompanied by ipsilateral localized extranodal organ invasion (IIE). Both the upper and lower lymph node regions of the diaphragm are invaded (III), and may be accompanied by localized extranodal organ invasion (IIIE) or splenic invasion (IIIS), or both (IIIES). Diffuse, disseminated extranodal organ or tissue invasion, with or without lymph node invasion (stage IV). The clinical data included sex, age, extranodal sites, bulky (the size of the primary tumor was bigger than 7.5 cm), cell of origin subtypes, first-line chemotherapy regimens, BCL-2, BCL-6, C-MYC, CD10, CD5, Ki-67, hepatitis B antigen, LDH, Alb (Albumin), PLT (Blood platelet), HB (Hemoglobin), FER, and β2 microglobulin. After excluding patients who lacked standard treatment (such as R-CHOP) cases and were younger than 18 years old, 402 patients who met the selection criteria were included in the analysis and entered the nomogram study cohort. Expression of biomarkers CD5, MYC, BCL-2, BCL-6, and Ki-67 was assessed using the respective antibodies. All histopathological slides were confirmed by at least 2 expert pathologists. The cut-off points of MYC, BCL-2 and BCL-6 protein were 40%, 50% and 50% positive staining of lymphoma cells, respectively. GCB or non-GCB phenotypes were determined according to the Hans algorithm. We randomly assigned 281 patients, in a 7:3 ratio, to the training group and 121 patients to the validation group.
Clinical indicators
Clinical characteristics assessed by clinical indicators include baseline characteristics (sex, age, extranodal sites, ECOG, bulky, cell of origin subtypes, first-line chemotherapy regimens, BCL-2, BCL-6, C-MYC, CD10, CD5, Ki-67, hepatitis B antigen, LDH, Alb, PLT, HB, FER, and β2 microglobulin). Response was assessed according to the International Working Group criteria. OS was defined as the survival time from initial diagnosis to the last follow-up or death.
Nomogram construction and validation
First, univariate Cox regression analysis was performed in the training group to screen out factors related to OS of patients with advanced DLBCL, and then multivariate Cox analysis was used to obtain independent prognostic factors affecting OS of patients with advanced DLBCL, which were then applied to the development of the nomogram. Internal validation was first performed, estimating the C-index by analyzing the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. Next, we constructed a calibration plot to determine whether the predicted and observed survival probabilities agree. This figure uses Bootstrap resampling (1000 resamples). Finally, we performed external validation in which the nomogram was used to evaluate each patient in the validation cohort and Cox regression analysis was performed using the total score of each patient as an independent factor. Regression analysis was then used to derive the C-index and calibration curve.
Statistical analysis
Survival curves were estimated by the Kaplan–Meier method and compared with log-rank tests stratified by prognostic factors. Nomogram was constructed based on Cox model parameter estimates in the training cohort. The selection of the final model was performed using a reverse stepwise descent selection procedure. Nomogram was constructed and validated according to Iasonos guidelines [20]. Statistical analyses were performed using the Hmisc, rms, survival ROC package in IBM SPSS Statistics (version 20.0) and R (version 4.2.1) (http://www.r-project.org/).
Result
Patient characteristics
This study included 402 patients with newly diagnosed stage III/IV DLBCL. Table 1 shows the clinical characteristics of 281 patients with stage III/IV DLBCL in the training group. The median survival time in the training group was 55 months. A total of 263 (65.4%) patients survived and 139 (34.6%) died, 87 of whom died from disease progression and 37 died from tumor-related complications, including 9 from infection-related disease and 6 from respiratory failure due to tumor compression of the mediastinum. Ten patients died of massive gastrointestinal hemorrhage due to tumor rupture, and 12 died due to cachexia. The final 15 patients died of non-tumor related diseases, including 3 patients who died of cerebral infarction, 4 patients who died of heart failure, 5 patients who died of severe pneumonia, and 3 patients who died of natural causes after recovery. According to MaxStat method, the optimal cut-off values of Age, LDH, Alb, PLT, HB, FER, and β2 microglobulin were 60 years old, 245U/L, 40 g/L, 100*10^9/L, 120 g/L, 130 μg/L, and 3.05 mg/L, respectively. There were 152 males (54.1%) and 129 females (45.9%). A total of 145 cases (51.6%) were less than 60 years old, and 136 cases (48.4%) were more than 60 years old at the first diagnosis. Of all patients in the training group who received first-line treatment, 202 (71.8%) received chemotherapy with R-CHOP, 35 (12.4%) received chemotherapy with CHOP, 13 (4.6%) received chemotherapy with R-DA-EPOCH, and 31 (11.2%) received other treatments. There were 260 cases (92.4%) with ECOG score 0–1 and 21 cases (7.6%) with ECOG score greater than 1. The number of extranodal involvement was 0–1 in 138 (49.1%) patients and 1 or more in 143 (50.9%) patients. There were 79 cases (28.1%) with large tumors at initial diagnosis. There were 134 cases (51.2%) of GCB subtype and 128 cases (48.9%) of non-GCB subtype. A total of 202 patients (72.7%) were treated with R-CHOP as first-line treatment. At initial diagnosis, 215 (76.5%) patients were positive for BCL-2, 232 (82.6%) patients were positive for BCL-6, 216 (76.9%) patients were positive for C-MYC, and 86 (30.6%) patients were positive for CD10. The 260(92.4%) patients had an ECOG score of 0–1. CD5 expression was positive in 92 (32.7%) patients, Ki-67 was low expressed in 75 (26.7%) patients and high expressed in 206 (73.3%) patients. Hepatitis B surface antigen was positive in 41 (14.6%) patients, and LDH was higher than normal value in 148 (52.7%) patients. After first-line treatment, 86 patients (30.6%) achieved CR (Complete remission),71 patients (25.2%) achieved PR(Partial remission), 55 patients (19.6%) achieved SD(Stable disease), and the remaining 69 patients (24.6%) achieved PD(Progressive disease).
Survival analysis, nomogram construction and internal validation
The patients in the nomogram development cohort (n = 402) were divided into training cohort (n = 281) and validation cohort (n = 121) according to the ratio of 7:3. Univariate analysis was performed to identify potential prognostic factors in the training cohort: age (≥ 60 vs < 60,p < 0.01), BCL-2 (positive vs negative, p = 0.02), C-MYC (positive vs negative, p < 0.001), CD5 (positive vs negative, p = 0.003), KI-67 (high expression vs low expression, p < 0.001), LDH (elevated vs normal, p = 0.02), PLT (elevated vs normal, p = 0.28), FER (elevated vs normal, p < 0.001), β2 microglobulin (elevated vs normal, p < 0.001), HB (elevated vs normal, p = 0.04), and Alb (elevated vs normal, p = 0.05). In multivariate analysis, Ki-67 (p < 0.001), LDH (p = 0.05), FER (p < 0.001) and β2 microglobulin (p < 0.001) were the independent risk factors related to the prognosis of patients (Table 2). Subsequently, ki-67, LDH, FER, and β2 microglobulin were used for nomogram construction (Fig. 1). The values on the variable axis attributed to a single case were located and a vertical line was drawn upward from the variable axis to determine the total number of points assigned to the patient, enabling an estimate of the OS rate on the survival axis. Based on the constructed nomogram, the total score was used to identify three discrete risk groups according to the X-tile: low-risk, intermediate risk, and high-risk. There was no crossover in the KM curve drawn according to the risk groups scored by the nomogram. The 5-year OS rates of low-risk group, intermediate high-risk group and high-risk group were 81.6%, 44.2% and 6%, respectively. (Fig. 2). Internal validation showed good agreement between the predicted values of the nomogram and the actual 3-year OS rate in the calibration curve (Fig. 3a). In the internal validation cohort, the C-index was 0.76 and the AUC was 0.828 (Fig. 3b).
Nomogram external validation
The nomogram was externally validated by the calibration plot in Fig. 3a and by calculating bootstrap C-index in an independent validation cohort of 121 patients. In the external validation step, the C-index of the nomogram for predicting 3-year OS was 0.74, indicating that it is a model with good discrimination. The calibration curve indicated a good nomogram (Fig. 4a), and the AUC was 0.803 (Fig. 4b).
Comparison of OS predictive accuracy between the nomogram and current staging or prognostic scoring systems
Both IPI low-risk and high-risk patients had a good prognosis stratification level (Fig. 5). For patients with stage III/IV DLBCL, the IPI score had poor stratification ability for patients with low-risk and low intermediate risk, and the 5-year survival rates were 61.5% and 55.8%, respectively. However, when the low-risk and low intermediate risk were included in our nomogram, the nomogram showed better stratification ability, and the 5-year survival rates were 93%, 34% and 6%, respectively (Fig. 6a). Similarly, for patients with stage III/IV DLBCL, the IPI score had poor stratification ability for high-intermediate and high-risk groups, with 5-year survival rates of 36.9% and 13.4%, respectively. However, when high-intermediate and high-risk groups were included in our nomogram, the nomogram showed better stratification ability. The 5-year survival rates of low, intermediate and high-risk patients were 52.9%, 30% and 6% (Fig. 6b), respectively. The C-index of the nomogram in the training cohort (0.72) was higher than that of the IPI (0.70).
Discuss
DLBCL is the most common type of non-Hodgkin lymphoma, with strong heterogeneity and poor prognosis [23]. About 60–70% of DLBCL patients are at stage III/IV at the time of initial diagnosis. Since the 1990s, IPI has become a standard tool for risk stratification and guiding therapy [24]. In the IPI score, stage is an important prognostic factor, and the higher the stage, the worse the prognosis. However, for advanced DLBCL patients with poor prognosis, IPI does not seem to show good prognostic stratification ability. At present, there is no prognostic tool for advanced-stage DLBCL. Therefore, the aim of this study is to develop a good prognostic model for advanced-stage DLBCL patients to accurately guide treatment.
The nomogram was designed to estimate the probability of 1, 3, and 5-year OS based on multivariate Cox proportional hazards model. The final nomogram model consisted of four variables from routine clinical practice: Ki-67, LDH, FER and β2 microglobulin. These four factors have been confirmed to have an impact on the prognosis of DLBCL patients in other studies. For Ki-67, it has been shown that high Ki67 index is an important adverse prognostic factor in DLBCL patients after the introduction of rituximab [25, 26]. For LDH, the standard prognostic score IPI has included LDH in the prognosis stratification of DLBCL patients, and high LDH indicates poor prognosis [18, 27].For β2 microglobulin, some studies have shown that β2 microglobulin can identify subsets with poor prognosis in intermediate-risk patients with DLBCL [28] and may improve NCCN-IPI score [29]. For ferritin, some studies have shown that the prognostic model incorporating ferritin has better prognostic stratification ability than IPI for DLBCL [30]. The DLBCL patients were scored according to the nomogram, and the risk group was divided according to the total score. According to the prognosis survival curve, the model had a good predictive function.
In IPI score, stage was an important prognostic factor, and stage III/IV accounted for 1 point [31]. The efficacy of IPI score in the stratification of prognosis will be affected. Our study shows the same conclusion. The total score of the nomogram was divided into three risk groups by X-tile software. There was no crossover in the KM curve drawn according to the risk groups scored by the nomogram, suggesting that the nomogram model has good prognostic discrimination ability in patients with advanced DLBCL. However, the prognosis stratification level of patients with low-risk and low-intermediate, high-intermediate and high-risk according to IPI score was poor, but the low-risk and low-intermediate, high-intermediate and high-risk groups were included in the nomogram for prognosis stratification, and the nomogram showed good prognosis stratification level. Therefore, our nomogram showed better prognostic stratification ability than IPI score for patients with advanced DLBCL.
This study still has several limitations. First, there is no uniform standard treatment for DLBCL patients in this study, which has R-CHOP, R-DA-EPOCH, or R-CHOP + X, which may cause some impact on the study. Second, this study was retrospective, which may have caused potential selection bias. Third, because there were some missing values of some indicators in this database, these indicators were not included in the construction of the model.
In conclusion, we have developed and externally validated a nomogram that can predict 5-year OS in advanced DLBCL in a highly accurate manner based on a large cohort of affected patients from endemic areas. The proposed nomogram showed a better discrimination level than IPI in the training set and provided individual risk assessment for DLBCL patients.
Data availability
The data set used and/or analyzed during the current study is available from the corresponding author on reasonable request.
References
Cultrera JL, Dalia SM. Diffuse large B-cell lymphoma: current strategies and future directions. Cancer Control. 2012;19(3):204–13.
Yang QP, et al. Subtype distribution of lymphomas in Southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol. 2011;6:77.
Zelenetz AD, et al. NCCN Guidelines® insights: B-Cell lymphomas, version 5.2021. J Natl Compr Canc Netw. 2021;19(11):1218–30.
Vitolo U, et al. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2016;27:v91–102.
Susanibar-Adaniya S, Barta SK. 2021 Update on Diffuse large B cell lymphoma: a review of current data and potential applications on risk stratification and management. Am J Hematol. 2021;96(5):617–29.
Coiffier B, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040–5.
Hawkes EA, Barraclough A, Sehn LH. Limited-stage diffuse large B-cell lymphoma. Blood. 2022;139(6):822–34.
Spinner MA, Advani RH. Current frontline treatment of diffuse large b-cell lymphoma. Oncology (Williston Park). 2022;36(1):51–8.
Poletto S, et al. Treatment strategies for patients with diffuse large B-cell lymphoma. Cancer Treat Rev. 2022;110: 102443.
Crump M, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–8.
He MY, Kridel R. Treatment resistance in diffuse large B-cell lymphoma. Leukemia. 2021;35(8):2151–65.
Moore DC, et al. New and emerging therapies for the treatment of relapsed/refractory diffuse large B-cell lymphoma. J Oncol Pharm Pract. 2022;28(8):1848–58.
Zelenetz A, et al. NCCN guidelines® insights: B-Cell Lymphomas, Version 5.2021. J Natl Compr Cancer Netw JNCCN. 2021;19(11):1218–30.
Major A, Smith SM. DA-R-EPOCH vs R-CHOP in DLBCL: How do we choose? Clin Adv Hematol Oncol. 2021;19(11):698–709.
Melchardt T, Egle A, Greil R. How I treat diffuse large B-cell lymphoma. ESMO Open. 2023;8(1): 100750.
Tokola S, et al. Significance of bulky mass and residual tumor-treated with or without consolidative radiotherapy-To the risk of relapse in DLBCL patients. Cancer Med. 2020;9(6):1966–77.
Durani U, Ansell S. CD5+ diffuse large B-cell lymphoma: a narrative review. Leuk Lymphoma. 2021;62(13):3078–86.
The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med, 1993. 329(14): 987–94.
Lin JY, et al. Clinicopathological features and prognostic factors of DLBCL. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2018;26(3):779–83.
Iasonos A, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–70.
Yuan SQ, et al. Development and validation of a nomogram to predict the benefit of adjuvant radiotherapy for patients with resected gastric cancer. J Cancer. 2017;8(17):3498–505.
Yang Y, et al. Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T-cell lymphoma, nasal-type: a multicenter study. Leukemia. 2015;29(7):1571–7.
Goldfinger M, Cooper DL. Refractory DLBCL: challenges and treatment. Clin Lymphoma Myeloma Leuk. 2022;22(3):140–8.
Martelli M, et al. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87(2):146–71.
Gaudio F, et al. High Ki67 index and bulky disease remain significant adverse prognostic factors in patients with diffuse large B cell lymphoma before and after the introduction of rituximab. Acta Haematol. 2011;126(1):44–51.
Tang YL, et al. BCL2/Ki-67 index predict survival in germinal center B-cell-like diffuse large B-cell lymphoma. Oncol Lett. 2017;14(3):3767–73.
Duan S, et al. Contrast-enhanced ultrasound parameters and D-Dimer: new prognostic parameters for diffuse large B-cell lymphoma. cancer manag res. 2022;14:2535–44.
Chen NC, et al. Beta2-microglobulin is a valuable marker and identifies a poor-prognosis subgroup among intermediate-risk patients with diffuse large B cell lymphoma. Clin Exp Med. 2023;23(7):3759–66.
Melchardt T, et al. A modified scoring of the NCCN-IPI is more accurate in the elderly and is improved by albumin and β2 -microglobulin. Br J Haematol. 2015;168(2):239–45.
Shen Z, et al. The addition of ferritin enhanced the prognostic value of international prognostic index in diffuse large B-Cell lymphoma. Front Oncol. 2021;11: 823079.
Ruppert AS, et al. International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood. 2020;135(23):2041–8.
Funding
This work was supported by Outstanding Young Scientific and Technological Talents Fund of Sichuan Province [grant number 2022JDJQ0059];The National Natural Science Foundation of China [grant number 82270198,82003196].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Mengdi Wan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
This study was approved by the Ethics committee of Sichuan Cancer Hospital with an Ethics committee approval number of 2020LP0022, and the confidentiality of patient data was guaranteed according to the requirements of the Ethics Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wan, M., Zhang, W., Huang, H. et al. Development and validation of a novel prognostic nomogram for advanced diffuse large B cell lymphoma. Clin Exp Med 24, 64 (2024). https://doi.org/10.1007/s10238-024-01326-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-024-01326-y