Abstract
The predictive value of allele frequency (AF) of BRAF V600E and TERT mutations in papillary thyroid carcinoma (PTC) remains controversial. We aimed to investigate the AF of BRAF V600E and TERT mutations in intermediate-to-high risk PTC and their association between tumor invasiveness, prognosis, and other mutations. Probe hybridization capture and high-throughput sequencing were used to quantitatively test 40 gene loci in 94 intermediate-to-high recurrence risk PTC patients, combined with clinical characteristics and follow-up for retrospective analysis. BRAF V600E mutation AF was linked to a increased risk of thyroid capsule penetration, recurrence, and concurrent mutations. Concurrent mutations could lead to a worse prognosis and increased invasiveness. TERT promoter mutation frequently accompanied other mutations and resulted in a poorer prognosis. However, there was no clear association between the TERT mutation AF and tumor invasiveness or recurrence. The sensitivity and specificity of predicting recurrence in intermediate-to-high risk PTC with BRAF V600E mutation AF > 28.2% were 60 and 80%. Although genetic alterations in PTC can differ among different ethnicities, the AF of BRAF V600E and TERT mutations may be similar. The AF of BRAF V600E has the potential to be a novel indicator in predicting PTC invasiveness and prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, there has been a global increase in the occurrence of thyroid cancer [1]. Papillary thyroid carcinoma (PTC) is the most frequent type of thyroid cancer, and the BRAF V600E mutation is the most frequent genetic mutation in PTC [2]. The BRAF gene encodes B-Raf protein kinase, which is a crucial member of the MAPK signaling pathway that regulates cell proliferation and differentiation [3]. Research suggests an association between PTC with BRAF V600E mutation and tumor volume, invasiveness and metastasis [4, 5]. However, it remains controversial whether BRAF V600E accurately reflects the malignancy and prognosis of the tumor [6,7,8]. The TERT gene encodes telomerase, which is a crucial enzyme for maintaining chromosomal telomere length. Activation of TERT gene mutations can sustain telomere length, leading to unlimited cell proliferation. Research has shown that mutations in the TERT gene are related to increased tumor size, invasiveness, lymph node metastasis and resistance to radiotherapy [9, 10]. However, the severity of PTC with BRAF V600E mutation can be variable, and patients with BRAF mutations may also present with different conditions, possibly related to the individual frequency of BRAF V600E in each patient [9]. Allele frequency (AF) is calculated by dividing the number of mutated molecules by the total number of wild-type molecules at a specific position in the genome [11, 12]. In this retrospective review of gene test results from Chinese patients with intermediate-to-high recurrence risk PTC [13], we analyzed and explored the impact of BRAF V600E and TERT mutation AF, aiming to identify reliable indicators that reflect the invasiveness and prognosis of PTC.
Materials and methods
Patients
A retrospective analysis was conducted on 106 patients with thyroid cancer who underwent genetic sequencing at the Department of Thyroid Surgery, The Second Hospital of Dalian Medical University, from November 2018 to December 2023. The inclusion criteria were: (1) postoperative pathology confirming PTC and (2) intermediate or high-risk stratification for PTC recurrence, stratified according to the ATA initial recurrence risk stratification [13]. 94 patients who met the inclusion criteria were included. The data collected included age, sex, stage, pathology, genetic testing results, and follow-up outcomes.
Second-generation sequencing
The gene testing was conducted by Beijing Genetron Health Co., Ltd. The Genetic testing loci was shown in Table 1. The steps of the testing process are as follows:
-
(1)
DNA Extraction: DNA concentration was measured using Qubit dsDNA HS Assay Kits (Thermo Fisher Scientific, Q32851), and RNA concentration was measured using Qubit RNA HS Assay Kits (Thermo Fisher Scientific, Q32852).
-
(2)
Reverse Transcription and PCR Amplification: To prepare the reaction system, take 0.2-mL PCR tubes equal to the number of TNA samples + 2. Total TNA 100 ng, make up to 7 µL with nuclease-free water. For RNA heat treatment, use the following reaction conditions: 80 °C for 10 min followed by 25 °C for 3 min. Then add 1μL of reverse transcriptase solution and 2μL of reverse transcriptase buffer solution to the reaction, mix well, and place on ice. Finally, set the PCR machine program with synthesis conditions. Perform PCR amplification by taking out the cDNA amplification primer mix, specific adapter, and polymerase mix, melting, mixing and following the temperature and time conditions of 25 °C for 10 min, 42 °C for 60 min, and 85 °C for 5 min.
-
(3)
Library Construction: To mix the magnetic beads, place them at room temperature for 30 min, vortex to disperse the beads, and slowly aspirate the solution. Next, prepare an 80% ethanol solution. Add 3μL of DNA library from pool 1 to pool 2 in a 3:20 ratio, shake well, let it stand at room temperature for 5 min, centrifuge briefly, place on a magnetic stand for about 5 min until the liquid clears, and discard the supernatant.
-
(4)
Library Quantification: The library concentration should be quantified using the Qubit fluorescence quantification system with the recommended DNA nucleic acid quantification reagent kit. Additionally, the library concentration and band size should be quantified using Agilent 2100/2200/4200. A target fragment size of around 180-300 bp is recommended, and the dimer proportion should not exceed 25% to be considered qualified. Sequencing should be performed on samples using the GENETRON S5 gene sequencer produced by Genergy Bio Technology. The BaseCaller software is utilized for base identification and base information statistics, and the TMAP software is used to align the sequencing results to the human reference genome sequence hg19 (GRCh37). Bam files and data statistics are generated for all samples on the chip.
-
(5)
Quality Control Conditions: For DNA samples: Average sequencing depth (Mean Depth) ≥ 5000x; BRAF V600 sequencing depth ≥ 2000x; TERT amplicon average sequencing depth ≥ 1000x. For RNA samples: The number of reads aligned to the target region ≥ 20,000; At least 3 out of 5 reference genes have a read count ≥ 50.
Statistical analysis
The analysis was performed using Statistical Package for Social Sciences (SPSS) version 29.0. Descriptive and frequency analyses were conducted, and the distribution was represented using frequencies, means, and standard deviations. Nonparametric tests or chi-square tests were employed for data that did not meet the assumption of normal distribution or had a small sample size, making normality tests impractical. Conversely, data conforming to normal distribution underwent analysis of variance or t tests. Logistic regression analyses were used to analyze AF between clinical statistics. The ROC curve was used to determine the optimal critical value. The confidence interval was based on 95%, and the level of statistical significance was p value < 0.05.
Results
This study included 94 patients with intermediate-to-high recurrence risk in PTC, with a male-to-female ratio of 1:1.35. The mean age was 43.99 ± 15.35 years, and the average follow-up time was 1.90 ± 1.75 years. No distant metastases were detected in patients during diagnosis or follow-up. Among these patients, 88 (93.62%) patients exhibited at least one genetic alteration. 78 (82.98%) patients had gene mutations, 11 (11.70%) patients had gene fusions, 1 (1.06%) patient had both gene mutation and fusion, and 6 (6.38%) patients showed no genetic abnormalities. The gene mutation events observed included BRAF V600E missense mutation (n = 75), TERT promoter mutation (n = 17), AKT1 missense mutation (n = 1), KRAS missense mutation (n = 1), TP53 missense mutation (n = 1), PIK3CA missense mutation (n = 2), and HRAS missense mutation (n = 1). The gene fusion events observed in this study included CCDC6-RET gene fusion (n = 7), NCOA4-RET gene fusion (n = 2), and ETV6-NTRK3 gene fusion (n = 2).
Association between AF of BRAF V600E mutation and gender/age
The BRAF V600E mutation occurred in 75 cases (76.5%) of PTC patients, with a male-to-female ratio of 1:1.27. The average age was (44.89 ± 15.03) years, and the mean BRAF V600E mutation AF was (19.36 ± 11.27) %. For males, the average of AF was (19.09 ± 10.60%), and for females, it was (19.58 ± 11.89%). The difference in BRAF V600E mutation AF between males and females was not statistically significant (p = 0.87). The age distribution of average BRAF mutation frequency is shown in Table 2, and the trend is illustrated in Fig. 1, indicating a rise-fall-rise pattern with peaks at ages 30–39 years and 70–79 years groups. According to ATA and AJCC guidelines [13], age 55 is a critical point for AJCC staging. Therefore, the study population was divided into ≥ 55 years and < 55 years groups, showing no statistically significant difference in BRAF mutation AF between the two groups (p = 0.28).
Association between AF of BRAF V600E mutation and extrathyroidal extension
Among PTC patients with BRAF V600E mutations, 18 cases (24%) showed extrathyroidal extension breaking the thyroid capsule, as shown in Table 3. The line graph in Fig. 2 shows the relationship between the number of cases with thyroid cancer invasion and the BRAF V600E mutation AF. A significant association was found between extrathyroidal extension and the increased AF of BRAF V600E mutation. (p = 0.002, OR = 1.100, OR (95%CI) = 1.037–1.166).
Association between AF of BRAF V600E mutation and recurrence
Among PTC patients with BRAF mutations, 10 cases (13.3%) experienced recurrence, as shown in Table 3, with an average follow-up time of (1.86 ± 1.26) years. A significant association was found between BRAF V600E mutation AF and thyroid cancer recurrence (p = 0.023, OR = 10.080, OR (95%CI) = 1.010–1.154). Figure 2 shows the line graph depicting the recurrence rate/BRAF V600E mutation AF. The receiver operating characteristic (ROC) curve was created using sensitivity on the y-axis and (1-specificity) on the x-axis. The area under the curve (AUC) was 0.717 (95% CI = 0.541–0.893), as shown in Fig. 3. The point with the highest Youden index was at a BRAF V600E mutation AF of 28.2% (sensitivity = 60.0%, specificity = 80.0%, Youden index = 0.40, accuracy=77.3%), as shown in Table 4. Table 5 shows that the risk of thyroid cancer recurrence is six times higher with a BRAF V600E mutation AF > 28.2% compared to AF ≤ 28.2%.
Association between AF of BRAF V600E mutation and combination with double/multiple loci mutations
Among PTC patients with BRAF mutations, 14 cases (18.67%) exhibited double/multiple loci mutations. These included 11 cases with BRAF + TERT mutations, 1 case with BRAF + KRAS mutation, 1 case with BRAF + TERT + PIK3CA mutations, and 1 case with BRAF + TERT + AKT1 mutations. The likelihood of double/multiple mutations significantly increased with the elevated BRAF gene mutation frequency (p = 0.006, OR = 1.088, OR (95%CI) = 1.024–1.156), as shown in Fig. 2, which depicts the relationship with BRAF V600E mutation AF. Compared to the group with a single BRAF mutation, the group with combined double/multiple BRAF mutations had a higher recurrence rate (p = 0.019, OR = 10.687, OR (95%CI) = 2.468–46.282), and a higher incidence of thyroid cancer breaking through the thyroid capsule (p < 0.001, OR = 16.562, OR (95%CI) = 4.178–65.662), as shown in Table 6.
Association between AF of TERT mutation and clinical statistics
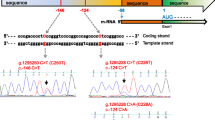
TERT promoter mutations were present in 17 cases (17.3%) of PTC patients, with a gender ratio of 1:1.13 and an average age of (61.41 ± 10.86) years. The average TERT mutation frequency was (46.71 ± 11.09) %, and all mutations were C228T missense mutation. As shown in Table 7, 1 patient had a single-point mutation, 13 patients had double-point mutations, 2 patients had triple-point mutations, and 1 patient had a TERT gene mutation combined with ETV6-NTRK3 gene fusion. 7 patients (41.18%) experienced recurrence with a mean follow-up of (2.65 ± 5.17) years. Patients who experienced recurrence all had TERT mutation frequencies greater than 45%. There were no statistically significant differences in TERT mutation AF between different genders and age groups. Increasing BRAF V600E mutation AF leading to a significant increase in TERT mutations (p = 0.002, OR = 1.116, OR (95%CI) = 1.042–1.197). Patients with TERT mutations have a significantly higher recurrence rate (p < 0.001, OR = 12.429, OR (95%CI) = 2.805–55.064) and an increased risk of tumor breakthrough of the capsule (p < 0.001, OR = 22.500, OR (95%CI) = 5.078–99.696), as shown in Table 8. There were no clear statistically significant differences in gender, age, thyroid capsule penetration, and recurrence among different AF of TERT mutation.
Gene fusion
11 cases (11.22%) were detected with gene fusions, with a sex ratio of 1:1.75 and a mean age of (38.09 ± 16.15) years. The gene fusions included 7 cases of CCDC6-RET fusion, 2 cases of NCOA4-RET fusion, and 2 cases of ETV6-NTRK3 fusion, as shown in Table 9. The CCDC6-RET fusion was found on chromosome 10, involving exon E1:E12 fusion. The NCOA4-RET fusion was found on chromosome 10, involving exon E8:E12 fusion. The ETV6-NTRK3 fusion was found on chromosomes 12 and 15, involving exon E4:E14 fusion. The remaining 10 cases did not exhibit any combined gene mutations. Out of the 11 cases of gene fusion patients with papillary thyroid carcinoma, only one case showed a combination of ETV6-NTRK3 fusion and TERT mutation. Among the gene fusion patients, two cases of NCOA4-RET fusion were of the Diffuse Sclerosing Variant of Papillary Thyroid Carcinoma (DSVPTC). Additionally, one patient with ETV6-NTRK3 gene fusion experienced recurrence.
Figures, tables and schemes
Discussion
The incidence of PTC is higher in females than in males, with a ratio of 2–3 to 1. However, in patients with intermediate-to-high recurrence risk PTC, females are only 1.36 times than males, suggesting that more males develop intermediate-to-high risk PTC. Some studies suggested that male is considered one of the risk factors for lymph metastasis in thyroid cancer [14]. We observed no significant differences in the allele frequencies of BRAF V600E and TERT mutations between males and females, which indicates that the severity of thyroid cancer in males may be associated with other factors. The occurrence rates of TERT mutations in thyroid cancer do not differ significantly among Asians, Europeans, and Americans [9, 10]. However, the occurrence rate of BRAF V600E mutations varies significantly across different races. The incidence of BRAF V600E mutation varies among different populations. In American patients with PTC, the incidence is approximately 50.8% [3]. In Korean patients, the incidence is around 72% [4], while in Japanese patients, it is about 38% [5]. In Italian patients, the incidence is around 38.1% [7], and in Saudi Arabian patients, it is about 59.5% [6]. In Chinese PTC patients, the incidence is approximately 72.4% [11], which is similar to the rate observed in our retrospective analysis (76.5%). Although BRAF V600E is considered a major driver in Chinese PTC populations, BRAF V600E AF in Chinese PTC patients is not significantly higher than in other regions and is even lower in our study. The reported BRAF V600E AF in PTC in other regions is approximately 26% in Canada [12] and around 27% in Italy [15]. While our study found that the AF of BRAF V600E in Chinese patients was approximately 19.36%, demonstrating inconsistency between its AF and incidence characteristics.
Gene mutations and fusions often do not occur simultaneously, exhibiting mutual exclusivity. Only 1 patient was found to have both TERT mutation and ETV6-NTRK3 fusion, but this patient's condition was not severe. Among the patients analyzed, the most common gene fusion was CCDC6-RET fusion (63.6%), followed by NCOA4-RET fusion (18.2%) and ETV6-NTRK3 fusion (18.2%), which may be related to a history of neck exposure [16, 17].
The AF of BRAF V600E mutation is suspected to be associated with metastasis in PTC, leading to higher tumor staging and poor outcome [5, 11, 12]. However, some studies have contradicted this claim, stating that the AF of BRAF V600E mutations does not significantly impact the prognosis and invasiveness of PTC [15, 18]. These discrepancies may be related to the diversity of populations in different countries or variations in the risk stratification of patients. Tumors form due to genomic instability in somatic cells, which can lead to the emergence of aggressive clones that can survive and outcompete other cells in the microenvironment. With the competition among cells, various genomic compositions (Allele Frequency) have emerged. The overall density of somatic mutations is relatively low, and this is considered the biological basis for the indolent clinical behavior observed PTC [18,19,20,21]. The efficacy of AF varies in different literature, which may be due to its unclear efficacy in low-risk thyroid cancer patients, while its effect becomes more distinct in intermediate-to-high-risk patients, clearly demonstrating its association with the disease. Therefore, we focused on intermediate-to-high-risk population to avoid interference from the large low-risk PTC population. In addition, genetic testing is a costly procedure, and although we found that BRAF V600E AF can predict patient prognosis, it is apparently unnecessary for low-risk PTC patients, since next generation sequencing techniques are mainly applicable to populations with intermediate-to-high risk recurrence patients. However, as this is a single-center, single-region, single-ethnicity study, it has certain limitations. Future research could include multiple populations, regions, and larger sample sizes to further investigate the overall impact of BRAF and TERT gene frequencies on disease. In addition, 94.1% of TERT mutation carriers had other types of genetic alterations, which may be due to the small sample size of TERT mutations. The lack of significant association between TERT promoter mutation AF and disease prognosis may be influenced by other mutation types, which requires further investigation.
We analyzed the impact of BRAF V600E mutation AF on tumor invasion, co-occurring mutations, and recurrence in patients with PTC in intermediate and high-risk recurrence categories. For each 1% increase in BRAF V600E mutation frequency, the risk of tumor invasion, recurrence, and co-occurring gene mutations increases by 10.0%, 8.0%, and 8.8%, respectively. The combination of BRAF mutation with other gene mutations significantly increases the risk of thyroid cancer invading the thyroid membrane and recurrence, with TERT mutations being the most common co-occurring mutation. When the frequency of the BRAF V600E mutation is greater than 28.2%, the risk of thyroid cancer recurrence increases sixfold compared to when the frequency is 28.2% or lower. Using AF of BRAF V600E mutation > 28.2% to predict the recurrence of intermediate and high risk PTC has a sensitivity of 60% and a specificity of 80%, making it a potential new indicator for predicting the risk of thyroid cancer recurrence.
The study indicates that BRAF mutations are the primary genetic mutation events in PTC, followed by TERT mutations, while other types occur less frequently. TERT mutations are associated with older age (average 61.41 years) and a higher AF (average 46.71%). Research indicates that TERT mutation is infrequent in children and teenagers [22], while combined with BRAF mutation, they contribute to increased malignancy in thyroid cancer [23, 24]. Our study suggested that TERT mutations are often accompanied by other mutations. The higher malignancy of TERT mutations in PTC may be due to the co-occurrence of other mutations [25]. The co-occurrence of BRAF mutations with TERT mutations is the most common scenario, and there is a positive correlation between the AF of BRAF V600E and the risk of co-occurring TERT mutations. According to extensive data reviewed by scholars, there appears to be no significant correlation between BRAF mutation and distant metastasis in PTC patients, while TERT mutation has been associated with distant metastasis. But our study identified a potential association between a high allele frequency of BRAF V600E and TERT mutation, which is consistent with the findings of previous research, BRAF mutations may lead to abnormal overexpression of the TERT promoter [26]. However, among the 94 patients we reviewed, none showed evidence of distant metastasis. Further research is needed to explore the relationship between BRAF, TERT mutations and distant metastasis [27].
Conclusions
Although the incidence of BRAF V600E mutation varies across different regions, the AF of BRAF V600E mutation is similar between Asian and Western patients. AF of BRAF V600E is positively correlated with invasiveness and the risk of recurrence. It may induce other gene mutations, such as TERT mutations, thereby enhancing the invasive capabilities of the tumor and leading to a poorer prognosis. The AF of BRAF V600E shows potential as a novel indicator for predicting tumor invasiveness and prognosis.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AF:
-
Allele frequency
References
Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020;16(1):17–29.
Carling T, Udelsman R. Thyroid cancer. Annu Rev Med. 2014;65(1):125–37. https://doi.org/10.1146/annurev-med-061512-105739.
Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–90.
Hong AR, Lim JA, Kim TH, et al. The frequency and clinical implications of the BRAF(V600E) mutation in papillary thyroid cancer patients in Korea over the past two decades. Endocrinol Metab (Seoul). 2014;29(4):505–13.
Nasirden A, Saito T, Fukumura Y, et al. In Japanese patients with papillary thyroid carcinoma, TERT promoter mutation is associated with poor prognosis, in contrast to BRAF (V600E) mutation. Virchows Arch. 2016;469(6):687–96.
Fugazzola L, Puxeddu E, Avenia N, et al. Correlation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: data from a multicentric Italian study and review of the literature. Endocr Relat Cancer. 2006;13(2):455–64.
Ito Y, Yoshida H, Maruo R, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J. 2009;56(1):89–97.
Tabriz N, Grone J, Uslar V, et al. BRAF V600E mutation correlates with aggressive clinico-pathological features but does not influence tumor recurrence in papillary thyroid carcinoma-10-year single-center results. Gland Surg. 2020;9(6):1902–13.
El Zarif T, Machaalani M, Nawfal R, et al. TERT promoter mutations frequency across race, sex, and cancer type. Oncologist. 2024;29(1):8–14. https://doi.org/10.1093/oncolo/oyad208.PMID:37462445;PMCID:PMC10769781.
Choi YS, Choi SW, Yi JW. Prospective analysis of TERT promoter mutations in papillary thyroid carcinoma at a single institution. J Clin Med. 2021;10(10):2179.
Wang Z, Tang P, Hua S, et al. Genetic and clinicopathologic characteristics of papillary thyroid carcinoma in the Chinese population: high Braf mutation allele frequency, multiple driver gene mutations, and ret fusion may indicate more advanced TN stage. Onco Targets Ther. 2022;9(15):147–57. https://doi.org/10.2147/OTT.S339114.PMID:35173448;PMCID:PMC8841610.
Abdulhaleem M, Bandargal S, Pusztaszeri MP, Rajab M, Greenspoon H, Krasner JR, Da Silva SD, Forest VI, Payne RJ. The impact of BRAF V600E Mutation allele frequency on the histopathological characteristics of thyroid cancer. Cancers. 2023;16(1):113.
American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid carcinoma. THYROID, Volume 26, Number 1, 2016; doi: https://doi.org/10.1089/thy.2015.0020.
Liu C, Xiao C, Chen J, et al. Risk factor analysis for predicting cervical lymph node metastasis in papillary thyroid carcinoma: a study of 966 patients. BMC Cancer. 2019;19(1):622.
Gandolfi G, Sancisi V, Torricelli F, et al. Allele percentage of the BRAF V600E mutation in papillary thyroid carcinomas and corresponding lymph node metastases: no evidence for a role in tumor progression. J Clin Endocrinol Metab. 2013;98(5):E934–42.
Seethala RR, Chiosea SI, Liu CZ, et al. Clinical and morphologic features of ETV6-NTRK3 translocated papillary thyroid carcinoma in an adult population without radiation exposure. Am J Surg Pathol. 2017;41(4):446–57.
Leeman-Neill RJ, Kelly LM, Liu P, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014;120(6):799–807.
Liu S, Zhang B, Zhao Y, et al. Association of BRAF V600E mutation with clinicopathological features of papillary thyroid carcinoma: a study on a Chinese population. Int J Clin Exp Pathol. 2014;7(10):6922–8.
Guerra A, Sapio MR, Marotta V, et al. The primary occurrence of BRAF(V600E) is a rare clonal event in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2012;97(2):517–24.
Masoodi T, Siraj AK, Siraj S, et al. Evolution and impact of subclonal mutations in papillary thyroid cancer. Am J Hum Genet. 2019;105(5):959–73.
Fugazzola L, Muzza M, Pogliaghi G, et al. Intratumoral genetic heterogeneity in papillary thyroid cancer: occurrence and clinical significance. Cancers (Basel). 2020;12:2.
Chakraborty D, Shakya S, Ballal S, Agarwal S, Bal C. BRAF V600E and TERT promoter mutations in paediatric and young adult papillary thyroid cancer and clinicopathological correlation. J Pediatr Endocrinol Metab. 2020;33(11):1465–74.
Vuong HG, Altibi AMA, Duong UNP, Hassell L. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clin Endocrinol (Oxf). 2017;87(5):411–7.
Matsuse M, Mitsutake N. TERT promoter mutations in thyroid cancer. Endocr J. 2023;70(11):1035–49.
Perera D, Ghossein R, Camacho N, et al. Genomic and Transcriptomic Characterization of Papillary Microcarcinomas With Lateral Neck Lymph Node Metastases. J Clin Endocrinol Metab. 2019;104:4889–99.
Liu R, Bishop J, Zhu G, et al. Mortality risk stratification by combining BRAF V600E and TERT promoter mutations in papillary thyroid cancer: genetic duet of BRAF and TERT promoter mutations in thyroid cancer mortality. JAMA Oncol. 2017;3(2):202–8.
Vuong HG, Altibi AM, Duong UN, et al. Role of molecular markers to predict distant metastasis in papillary thyroid carcinoma: promising value of TERT promoter mutations and insignificant role of BRAF mutations-a meta-analysis. Tumour Biol. 2017;39(10):1010428317713913.
Acknowledgements
This research was funded by the “1+X” project (2022LCJSGC05 and 2022MDTQL02) provided by the Second Hospital of Dalian Medical University.
Funding
This research was funded by the “1 + X” project (2022LCJSGC05 and 2022MDTQL02) provided by the Second Hospital of Dalian Medical University.
Author information
Authors and Affiliations
Contributions
JH, GW and YZ contributed to conceptualization; JH, JW and YZ contributed to data curation; JW and GW contributed to formal analysis; YZ contributed to funding acquisition; JW, JX and JW contributed to investigation; JH, GW and YZ contributed to methodology; GW and YZ contributed to project administration; JX and YZ contributed to resources; JW contributed to software; GW contributed to supervision; JX and GW contributed to validation; JW contributed to visualization; JH contributed to writing—original draft; JH, YZ and GW contributed to writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was supported by the Ethics Committee of the Second Hospital of Dalian Medical University.
Consent for publication
Not applicable.
Informed Consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, J., Wang, J., Xv, J. et al. Genetic alterations and allele frequency of BRAF V600E and TERT mutation in papillary thyroid carcinoma with intermediate-to-high recurrence risk: a retrospective study. Clin Exp Med 24, 76 (2024). https://doi.org/10.1007/s10238-024-01320-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-024-01320-4