Abstract
Thymic stromal lymphopoietin (TSLP) is critical in developing allergic responses, including atopic dermatitis (AD). We systematically reviewed the literature to complete a meta-analysis to quantitatively summarize the levels of serum TSLP in AD. The study was prospectively registered in the PROSPERO database (ID = CRD42021242628). The PUBMED, SCOPUS, and Cochrane Library databases were reviewed, and original articles investigating serum TSLP in AD patients were included. Differences in TSLP levels of AD patients and controls were summarized by standardized mean differences (SMD) using a random effects model. Study quality was assessed by applying the Newcastle‒Ottawa Scale. Fourteen studies, which included 1,032 AD patients and 416 controls, were included. Meta-analysis showed that TSLP levels were significantly higher in the AD group than in the control group (SMD = 2.21, 95% CI 1.37–3.06, p < 0.001). Stratification by geographical region, age, disease severity, TSLP determination method, sample size, and study quality revealed significantly elevated TSLP levels in European AD patients (SMD = 3.48, 95% CI 1.75–5.21, p < 0.0001), adult AD patients (SMD = 4.10, 95% CI 2.00–6.21, p < 0.0001), child AD patients (SMD = 0.83, 95% CI 0.08–1.59, p = 0.031), and all severity groups with AD compared with the control group (mild: SMD = 1.15, 95% CI 0.14–2.16, p = 0.025; moderate: SMD = 2.48, 95% CI 0.33–4.62, p = 0.024; and severe: SMD = 8.28, 95% CI 4.82–11.74, p = 2.72e−6). Noticeably, adults showed higher serum TSLP levels than children with AD, and serum TSL levels increased according to AD severity. In conclusion, our meta-analysis demonstrates that circulating TSLP levels are elevated in patients with AD. Future studies are warranted to further elucidate the sources of heterogeneity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atopic dermatitis (AD) is one of the most prevalent chronic relapsing inflammatory skin diseases. It usually develops in childhood and may persist into adulthood; less frequently, it starts in midlife or late life, and both sexes are affected [1, 2]. The prevalence and incidence of AD have increased over the past several decades [3]. Prevalences of 15–20% among children and up to 10% among adults have been reported, making AD the 15th most common nonfatal disease [4]. However, the prevalence of AD varies among races and ethnic groups [5].
AD is characterized by recurrent, pruritic, localized eczema, often with seasonal fluctuations. Many patients also have allergic asthma, allergic rhinoconjunctivitis, food allergies, and other immediate hypersensitivity (type 1) allergies [1, 2]. The clinical diagnosis of AD is based on the morphologic features and distribution of skin lesions, associated clinical signs, and a characteristic medical history [6]. A list of 23 clinical signs and symptoms of AD was published by Hanifin and Rajka in 1980 and is still used as a clinical research benchmark. AD severity can be quantitated with the Eczema Area and Severity Index (EASI), the Scoring Atopic Dermatitis (SCORAD) scale, and the Six Areas Six Sign AD (SASSAD) [7]. However, they all have high inter- and intra-observer variation.
AD pathogenesis results from complex interactions among genetic and environmental factors, skin barrier dysfunction, microbial imbalance, immune dysregulation, and environmental triggers of skin inflammation. Thymic stromal lymphopoietin (TSLP) is an IL-7–like cytokine produced by keratinocytes, although TSLP can also be produced by airway smooth muscle cells [8], human DCs [9], mast cells [10], human monocytes [9], macrophages and granulocytes [11]. TSLP exerts its biological activities by binding to a heterodimeric receptor complex consisting of the interleukin-7 receptor α chain (IL-7Rα) and the TSLP receptor chain (TSLPR) [12, 13]. TSLP is highly expressed in the epidermis of lesioned human AD skin.
TSLP can potently activate immature myeloid dendritic cells, which subsequently prime CD4 + T cells to produce allergy-promoting cytokines (such as interleukin (IL)-4, IL-5, IL-13, and TNF-α) and induce the production of TH2-attracting chemokines (CCL22 and CCL17) [10, 14]. TSLP affects several mast cell functions, including growth, survival, and mediator release [15]. Transgenic TSLP expression in keratinocytes results in AD-like skin inflammation [16]. Nevertheless, TSLP receptor-deficient mice are protected from developing allergic skin [17].
TSLP, expressed by the barrier-defective epidermis, is released into the systemic circulation [18]. Hence, several studies have focused on the relationship between serum TSLP levels and AD, concluding that TSLP levels are altered in this disease [19,20,21,22,23,24,25,26,27,28,29]. However, some studies showed that AD patients present similar serum TSLP levels compared to healthy controls [30,31,32]. Therefore, the association between TSLP levels and AD is still uncertain. To settle these controversial issues, this meta-analysis was performed to evaluate the relationship between serum TSLP levels and AD patients.
Materials and methods
This systematic review was conducted using a prospective protocol based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA) statement [33]. Details of the protocol were registered on Prospero (ID = CRD42021242628). Two authors were independently involved in the study selection (LZ and MAM), data extraction (MMG and MAM), and quality assessment (LZ and MMG), and disagreements were resolved by discussion with a third author (RP) if a consensus could not be reached. In this meta-analysis, ethical approval was unnecessary, as all the data were based on previously published studies.
Literature search strategy
We searched the PubMed, Scopus, and Cochrane Library databases for articles published from their inception until March 2021. The search strategy for each database is detailed in Supplementary Table 1. Titles and abstracts identified by the search were screened, and then full texts of selected articles were reviewed. All references cited were also reviewed to identify additional studies not indexed by the electronic databases.
Eligibility criteria
We included any peer-reviewed English language article that examined the relationship between serum TSLP levels and AD and used a case‒control, cross-sectional, cohort, or randomized control trial (RCT) study design. Any criteria for AD diagnosis were accepted. All studies had to measure serum TSLP levels, report mean differences with standard deviation, and have sufficient data for computation. Studies were excluded if they did not contain objective data on serum TSLP levels. We recruited data only from the full-published paper, and meeting and conference abstracts were excluded.
Data abstraction
We extracted information from each study using a predefined data extraction form designed for this review. For each eligible study, the following information was extracted: first author’s name, year of publication, journal, country, number of cases and controls with levels of TSLP measured, age, severity in the AD group, and mean and standard deviation of serum TSLP level (pg/mL). In most studies, the mean and deviation were obtained, but in several studies, only the median values and quartiles were reported. Therefore, when the original data were median values and quartiles, we transformed and calculated the data to gain the appropriate values according to the method recommended by Hozo et al. [34]. Furthermore, some studies applied WebPlotDigitizer 4.4 software to digitize and extract the data from the scatter diagrams. If important original data were unavailable in some articles, we contacted the corresponding author by e-mail to obtain further details.
Study quality assessments
The Newcastle‒Ottawa Scale was used to assess the quality of nonrandomized studies in the review. The evaluation comprised three broad perspectives and used the following star rating system: the selection of study groups (4 stars), the comparability of the groups (2 stars), and the ascertainment of the exposure or outcome of interest (3 stars). A study was graded as low, moderate, or high quality for scores of 0–3, 4–6, and 7–9 stars, respectively.
Statistical analysis
We conducted meta-analyses using Stata/BE, version 17 (StataCorp LP, College Station, TX). The first meta-analysis compared the mean concentrations of serum TSLP between participants with AD and those without AD. The standardized mean difference (SMD) was chosen as the summary statistic. For all analyses, we chose a random-effects model using the DerSimonian‒Laird method. To assess the degree of heterogeneity across studies, we used the I2 statistic and P value of the χ2 squared test. I2 values of 25%, 50%, and 75% were considered to indicate low, moderate, and high heterogeneity, respectively. To examine potential sources of heterogeneity in the meta-analysis, subgroup analysis was performed using the following variables: geographical region, age, severity of disease, TSLP-determined method, sample size, and study quality. A sensitivity test was performed to assess each study's influence on the SMD by omitting each study individually and deleting the studies with imputed data. To examine possible heterogeneities in the meta-analysis, a meta-regression analysis was performed using the following variables: publication year, mean age, proportion of males, sample size, and NOS. We used a funnel plot and Egger’s linear regression test to assess publication bias. All p values were two-sided, and the alpha was set at 0.05.
Results
Study selection and characteristics
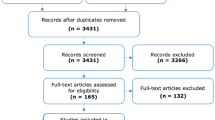
The initial search in the electronic PubMed, Scopus, and Cochrane Library (2002–2021) databases identified 795 studies. After screening, a total of 14 studies were included in our qualitative analysis; the results of the study selection process and reasons for exclusion are summarized in Fig. 1.
The 14 selected studies were published between 2008 and 2020 and originated from 11 different countries; seven studies were from Europe [19, 21, 23,24,25,26, 31], five studies were from Asia [22, 27, 29, 30, 32], one study was from America [28] and one study was from Africa [20]. Six studies included only children [20, 22, 26, 28,29,30], four studies included only adults [19, 21, 25, 32], and four studies included both children and adults [23, 24, 27, 31]. The proportion of male patients ranged from 33 to 80%, and the proportion male controls ranged from 30 to 71%. The sample sizes ranged from 9 to 165 patients and from 9 to 87 control participants (see Table 1).
The control groups were composed of healthy individuals [19,20,21,22,23,24,25,26,27, 29,30,31,32] or nonatopic patients [28]. Ten of the 14 included observational studies used predefined criteria for AD: 9 studies used Hanifin and Rajka’s criteria[20,21,22, 24,25,26,27, 31, 32], and one study used the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire criteria [30]; the rest of the studies did not report the diagnostic criteria used for patients with AD [19, 23, 28, 29]. Eleven studies determined the TSLP concentration in serum using ELISA [19,20,21,22,23,24, 26, 29,30,31,32], and 3 used a multiplex immunoassay [25, 27, 28]. The main characteristics of the 14 observational studies included in the review are summarized in Table 1.
Meta-analysis of serum TSLP levels in AD patients compared to controls
Of the 14 articles included in the meta-analysis, the serum TSLP levels (mean ± SD) could be extracted directly from 8 articles [19,20,21,22, 24, 25, 29, 32], whereas WebPlotDigitizer was used to digitize the graphical data from the other six articles [23, 26,27,28, 30, 31]. The TSLP levels were significantly higher in the AD group than in the control group (SMD = 2.21, 95% CI 1.37–3.06, p < 0.001) (Fig. 2). The total heterogeneity was very high in the meta-analysis (I2 = 97.46%). However, the sensitivity analysis showed that no individual study significantly affected the pooled SMD, indicating that the results of this meta-analysis were robust (Fig. 3).
The heterogeneity of each group after stratification was still very high, possibly because we measured the magnitude of heterogeneity but not the direction of effect sizes observed in the subgroups. SMDs were in the same positive direction in all of the studies, whereas there was a difference in the magnitudes of SMDs among individual studies (Fig. 2). Other unknown factors affecting heterogeneity may have contributed to the relationship between serum TSLP levels and AD.
In addition, stratification by geographical region of the studies showed significantly elevated TSLP levels in AD subjects in European populations (SMD = 3.48, 95% CI 1.75–5.21, p = 8.15e−5); in Asian populations, no difference was found. When grouped by age, children (SMD = 0.83, 95% CI 0.08–1.59, p = 3.11e−2) and adults (SMD = 4.10, 95% CI 2.00–6.21, p = 1.31e−4) showed significant differences. A greater effect on the TSLP levels was observed in adults, whereas no effect was found in the case of two studies that analyzed adults and children together Table 2.
In some studies, it was possible to group AD subjects by mild, moderate, and severe severity according to predefined scales, and we found higher serum levels of TSLP in the active patient groups than in the control groups for each severity level (Mild: SMD = 1.15, 95% CI 0.14–2.16; moderate: SMD = 2.48, 95% CI 0.33–4.62 and severe: SMD = 8.28, 95% CI 4.82–11.74). Interestingly, serum TSL levels increased according to the severity of AD.
The serum TSLP levels were determined by using ELISA or multiplex assays; in both cases, an effect of higher TSLP levels was observed in subjects with AD compared to in control participants (ELISA method: SMD = 1.21, 95% CI 0.55–1.87; and multiplex immunoassay method: SMD = 4.68, 95% CI 1.61–7.76). However, higher TSLP levels were observed in the studies that used the multiplex immunoassay method. The sample size of the groups in each study did not show an impact on the effect of the serum concentration of TSLP, nor did the quality of the study measured through the NOS scale.
Metaregression analysis.
To investigate whether the continuous variables, including the publication year of each study, NOS score of each study, mean age, and proportion of male AD subjects, had potential moderating effects on the pooled SMD, we performed a random-effects meta-regression analysis. The meta-regression analysis showed that the mean age (p = 0.0273) and proportion of males (p = 0.0057) among AD subjects (Fig. 4A, B), but not publication year (p = 0.5313) or sample size (p = 0.1819), had a significant impact on heterogeneity in the meta-analysis of TSLP levels.
Meta-regression analysis and publication bias analysis. A Bubble plot for random-effects meta-regression with mean age as a study-level covariate in all AD subjects. B Bubble plot for random-effects meta-regression with % of male as study-level covariate on all AD subjects, the size of a bubble is in proportion to the sample size of the corresponding study. C The trim-and-fill method imputed six missing studies to make the funnel plot symmetrical
Publication bias
The funnel plot asymmetry and Egger's regression results (P < 0.0001) indicated that there was publication bias. Therefore, the “trim and fill” method was used to adjust for bias. Compared to the previous pooled effect size (SMD = 2.21, 95% CI 1.37–3.06), the pooled SMD after adjustment (SMD = 0.762, 95% CI 0.221–1.744) remained significant, although the increased level of TSLP expression was reduced. This suggested that the meta-analysis results remained valid, although publication bias must be considered (Fig. 4C).
Study quality assessment
Two articles were considered high quality (NOS score, 7–9) [20, 25], and 11 were considered moderate quality (NOS score, 4–6) [21,22,23,24, 26,27,28,29,30,31,32], thus indicating that most studies had a low risk of bias. The study quality assessment scores for observational studies are shown in Table 2. For participant selection, three studies described the representativeness of cases, one study used hospital-based controls, and two studies provided minimal or no description of where the controls were sourced. For the comparability of groups, eight studies did not match or adjust for any potential confounders [19, 21,22,23, 28,29,30, 32], and six studies matched or adjusted for only 1 factor [20, 24,25,26,27, 31]. For the ascertainment of exposure, all studies used an objective measure of TSLP determination, and the same method was used for cases and controls, but no studies provided nonresponse rates or described nonrespondents (Tables 3, 4).
Discussion
TSLP is a promising therapeutic target that plays a critical role in the pathogenesis of AD; therefore, in this systematic review and meta-analysis, we set out to clarify whether serum TSLP in AD patients differs from controls by analyzing the published data available to date [35]. Our meta-analysis showed that serum TSLP levels are elevated in subjects with AD and are higher in adults than in children. In addition, higher serum levels were found in studies with subjects with severe AD and in the European population, in agreement with recent research advancements indicating that AD is a complex disease characterized by different subtypes/phenotypes based on age, disease chronicity, and ethnicity [36]. Interestingly, the Asian population with AD does not present elevated levels of TSLP (SMD = 0.71, 95% CI − 0.07 to 1.49, p = 0.076), which could be partly related to the fact that the Asian AD phenotype presents a blended phenotype between that of European-American patients with AD and those with psoriasis; furthermore, this population shows increased epidermal hyperplasia, greater TH17/TH22 and lower TH1 skewing, and comparable TH2 activation [37, 38].
Our analysis showed that children with AD have lower levels of serum TSLP than adults with AD. This could be explained because adults with AD have an increased frequency of IL-22–producing CD4 and CD8 T cells within the skin-homing population compared with children, [39] and these cells are involved in chronic changes in epidermal hyperplasia, which is primarily observed in adults [40].
The current study is the first meta-analysis to clarify alterations involving serum TSLP in AD patients. However, this study had certain limitations. First, substantial heterogeneity among the studies included in this meta-analysis should be noted. We used meta-regression to compare serum TSLP levels between AD patients and healthy controls to explore whether the source of heterogeneity was derived from sex, age, sample size, year of publication, and disease activity; the meta-regression showed that age and sample size affected between-study variation. However, no statistical significance was found with sample size, year of publication, and disease activity (p value of meta-regression > 0.05). However, the conclusion that there is no relationship between the above factors and inherently high heterogeneity cannot be drawn arbitrarily because of the lack of data regarding disease activity and duration in the included studies. Although the source of heterogeneity was difficult to determine, the ethnicity, treatment, and other factors may have affected the heterogeneity of the included studies.
Due to the lack of data, we did not analyze the correlation between TSLP levels and other disease parameters, such as the severity index according to the objective SCORAD index, total serum IgE levels, serum cytokines, or chemokines. Owing to these limitations, the results of this meta-analysis should be interpreted carefully.
In addition to strategies aimed at neutralizing the functions of TSLP in Atopic Dermatitis (AD) [41], recent research has pursued promising new therapeutic avenues, explored in preclinical trials using murine models. Among these, the topical application of calcitriol, an active form of Vitamin D, has the potential to ameliorate AD symptoms by restoring the dysfunctional epidermal and tight junction barriers frequently associated with this condition [42]. Additionally, the antimicrobial peptide derived from Insulin-like Growth Factor-Binding Protein 5 (AMP-IBP5) has demonstrated potential in modulating the cutaneous inflammatory environment [43]. This spectrum of emerging therapeutic options emphasizes the vital role of ongoing research in AD and highlights the necessity for a comprehensive approach that addresses not only the symptoms but also the underlying causes of this intricate disease.
Tezepelumab is a human monoclonal antibody that targets circulating TSLP. A clinical trial showed that patients with moderate to severe AD achieved numerical improvements over placebo when treated with tezepelumab; however, these improvements were not statistically significant [41]. However, two isoforms of TSLP, short and long isoforms, have been described; the main isoform expressed during steady-state conditions is the short form of TSLP, whereas the long form of TSLP is upregulated in inflammatory conditions [44]. However, since the expression patterns and biological properties of these two different isoforms of TSLP seem to be distinct, these two TSLP isoforms should be analyzed separately in future studies. This is highlighted by findings in asthma research, where it was observed that the asthma-associated long TSLP isoform negatively regulates the secretion of IgA, potentially impacting the surveillance of mucosal surfaces detrimentally in this condition [45].
Conclusion
Our meta-analysis demonstrates that serum TSLP levels are high in AD patients compared with non-AD subjects. Additionally, serum TSLP levels in AD adults are higher than those in AD children and increase according to AD severity. Further studies are necessary to elucidate how TSLP directly contributes to the pathogenesis of AD.
Availability of data and materials
The raw materials can be requested by communication with corresponding author.
References
Stander S. Atopic dermatitis. N Engl J Med. 2021;384(12):1136–43. https://doi.org/10.1056/NEJMra2023911.
Sroka-Tomaszewska J, Trzeciak M. Molecular mechanisms of atopic dermatitis pathogenesis. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22084130.
Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl 1):8–16. https://doi.org/10.1159/000370220.
Laughter MR, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017. Br J Dermatol. 2021;184(2):304–9. https://doi.org/10.1111/bjd.19580.
Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(5):449–55. https://doi.org/10.1016/j.anai.2018.11.015.
Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–51. https://doi.org/10.1016/j.jaad.2013.10.010.
Chopra R, Vakharia PP, Sacotte R, et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol. 2017;177(5):1316–21. https://doi.org/10.1111/bjd.15641.
Zhang K, Shan L, Rahman MS, et al. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L375–82. https://doi.org/10.1152/ajplung.00045.2007.
Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol. 2011;187(3):1207–11. https://doi.org/10.4049/jimmunol.1100355.
Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–80. https://doi.org/10.1038/ni805.
Ying S, O’Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181(4):2790–8. https://doi.org/10.4049/jimmunol.181.4.2790.
Park LS, Martin U, Garka K, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192(5):659–70. https://doi.org/10.1084/jem.192.5.659.
Pandey A, Ozaki K, Baumann H, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1(1):59–64. https://doi.org/10.1038/76923.
Liu YJ, Soumelis V, Watanabe N, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. https://doi.org/10.1146/annurev.immunol.25.022106.141718.
Saluja R, Zoltowska A, Ketelaar ME, Nilsson G. IL-33 and thymic stromal lymphopoietin in mast cell functions. Eur J Pharmacol. 2016;778:68–76. https://doi.org/10.1016/j.ejphar.2015.04.047.
Yoo J, Omori M, Gyarmati D, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202(4):541–9. https://doi.org/10.1084/jem.20041503.
Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202(6):829–39. https://doi.org/10.1084/jem.20050199.
Demehri S, Liu Z, Lee J, et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6(5):e123. https://doi.org/10.1371/journal.pbio.0060123.
Alysandratos KD, Angelidou A, Vasiadi M, et al. Increased affected skin gene expression and serum levels of thymic stromal lymphopoietin in atopic dermatitis. Ann Allergy Asthma Immunol. 2010;105(5):403–4. https://doi.org/10.1016/j.anai.2010.09.017.
Genedy R, Farid C, Saad E. Serum thymic stromal lymphopoietin in pediatric atopic patients and its relation to the development of the atopic March. J Egypt Womens Dermatol Soc. 2016;13(3):180–6. https://doi.org/10.1097/01.EWX.0000482855.26057.b6.
Jaworek AK, Szafraniec K, Zuber Z, Wojas-Pelc A, Jaworek J. Interleukin 25, thymic stromal lymphopoietin and house dust mites in pathogenesis of atopic dermatitis. J Physiol Pharmacol. 2020. https://doi.org/10.26402/jpp.2020.2.14.
Lee EB, Kim KW, Hong JY, et al. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr Allergy Immunol. 2010;21(2 Pt 2):e457–60. https://doi.org/10.1111/j.1399-3038.2009.00919.x.
Mocsai G, Gaspar K, Dajnoki Z, et al. Investigation of skin barrier functions and allergic sensitization in patients with hyper-IgE syndrome. J Clin Immunol. 2015;35(7):681–8. https://doi.org/10.1007/s10875-015-0200-2.
Nygaard U, Hvid M, Johansen C, et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol. 2016;30(11):1930–8. https://doi.org/10.1111/jdv.13679.
Thijs JL, Strickland I, Bruijnzeel-Koomen C, et al. Moving toward endotypes in atopic dermatitis: Identification of patient clusters based on serum biomarker analysis. J Allergy Clin Immunol. 2017;140(3):730–7. https://doi.org/10.1016/j.jaci.2017.03.023.
Uysal P, Birtekocak F, Karul AB. The relationship between serum TARC, TSLP and POSTN Levels and childhood atopic dermatitis. Clin Lab. 2017;63(7):1071–7. https://doi.org/10.7754/Clin.Lab.2017.161107.
Wang S, Zhu R, Gu C, et al. Distinct clinical features and serum cytokine pattern of elderly atopic dermatitis in China. J Eur Acad Dermatol Venereol. 2020;34(10):2346–52. https://doi.org/10.1111/jdv.16346.
Yao W, Zhang Y, Jabeen R, et al. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013;38(2):360–72. https://doi.org/10.1016/j.immuni.2013.01.007.
Byeon JH, Yoon W, Ahn SH, et al. Correlation of serum interleukin-31 with pruritus and blood eosinophil markers in children with atopic dermatitis. Allergy Asthma Proc. 2020;41(1):59–65. https://doi.org/10.2500/aap.2020.41.190016.
Lee E, Lee SH, Kwon JW, et al. Atopic dermatitis phenotype with early onset and high serum IL-13 is linked to the new development of bronchial hyperresponsiveness in school children. Allergy. 2016;71(5):692–700. https://doi.org/10.1111/all.12844.
Mihaly J, Gericke J, Lucas R, et al. TSLP expression in the skin is mediated via RARgamma-RXR pathways. Immunobiology. 2016;221(2):161–5. https://doi.org/10.1016/j.imbio.2015.09.013.
Nakamura K, Tsuchida T, Tsunemi Y, Saeki H, Tamaki K. Serum thymic stromal lymphopoietin levels are not elevated in patients with atopic dermatitis. J Dermatol. 2008;35(8):546–7. https://doi.org/10.1111/j.1346-8138.2008.00518.x.
Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. https://doi.org/10.1136/bmj.n160.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. https://doi.org/10.1186/1471-2288-5-13.
Adhikary PP, Tan Z, Page BDG, Hedtrich S. TSLP as druggable target—a silver-lining for atopic diseases? Pharmacol Ther. 2021;217: 107648. https://doi.org/10.1016/j.pharmthera.2020.107648.
Nomura T, Wu J, Kabashima K, Guttman-Yassky E. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8(6):1840–52. https://doi.org/10.1016/j.jaip.2020.02.022.
Noda S, Suarez-Farinas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136(5):1254–64. https://doi.org/10.1016/j.jaci.2015.08.015.
Wen HC, Czarnowicki T, Noda S, et al. Serum from Asian patients with atopic dermatitis is characterized by T(H)2/T(H)22 activation, which is highly correlated with nonlesional skin measures. J Allergy Clin Immunol. 2018;142(1):324–8. https://doi.org/10.1016/j.jaci.2018.02.047. (e11).
Czarnowicki T, Esaki H, Gonzalez J, et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)(+) TH2/TH1 cell imbalance, whereas adults acquire CLA(+) TH22/TC22 cell subsets. J Allergy Clin Immunol. 2015;136(4):941–51. https://doi.org/10.1016/j.jaci.2015.05.049. (e3).
Leung DY. Atopic dermatitis: age and race do matter! J Allergy Clin Immunol. 2015;136(5):1265–7. https://doi.org/10.1016/j.jaci.2015.09.011.
Simpson EL, Parnes JR, She D, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: a randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80(4):1013–21. https://doi.org/10.1016/j.jaad.2018.11.059.
Umehara Y, Trujillo-Paez JV, Yue H, et al. Calcitriol, an active form of vitamin D3, mitigates skin barrier dysfunction in atopic dermatitis NC/Nga mice. Int J Mol Sci. 2023;11:12. https://doi.org/10.3390/ijms24119347.
Nguyen HLT, Peng G, Trujillo-Paez JV, et al. The antimicrobial peptide AMP-IBP5 suppresses dermatitis-like lesions in a mouse model of atopic dermatitis through the low-density lipoprotein receptor-related protein-1 receptor. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms24065200.
Fornasa G, Tsilingiri K, Caprioli F, et al. Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. J Allergy Clin Immunol. 2015;136(2):413–22. https://doi.org/10.1016/j.jaci.2015.04.011.
van Heerden D, van Binnendijk RS, Tromp SAM, et al. Asthma-associated long TSLP inhibits the production of IgA. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22073592.
Acknowledgements
M.M.G. and L.C.Z. acknowledge fellowships from CONAHCYT, while R.P.P. and M.A.M. acknowledge support from SNI-CONAHCYT.
Funding
The Universidad Nacional Autónoma de México financed this work through DGAPA-PAPIIT Grant number IN219121 (awarded to R.P.P.) and IA208423 (awarded to M.A.M.).
Author information
Authors and Affiliations
Contributions
LCZ and MAM were independently involved in the study selection, MMG and MAM data extraction, LCZ and MMG quality assessment. RPP and MAM performed the statistical analyzes and wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate
No approval from research ethics committees was required to achieve the objectives of this study, as it involved a systematic review and meta-analysis of previously published articles.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Reyes, M.M., Zumaya-Pérez, L.C., Pastelin-Palacios, R. et al. Serum thymic stromal lymphopoietin (TSLP) levels in atopic dermatitis patients: a systematic review and meta-analysis. Clin Exp Med 23, 4129–4139 (2023). https://doi.org/10.1007/s10238-023-01147-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01147-5