Abstract
There have been few studies comparing the clinical characteristics and outcomes of SARS-CoV-2 pneumonia in individuals with and without moderately to severely immunocompromised conditions. We reviewed adult patients with SARS-CoV-2 infection who had radiologic evidence of pneumonia at a tertiary hospital in Seoul, South Korea, from February 2020 to April 2022. Moderately to severely immunocompromised status was defined as medical conditions or treatments that resulted in increased risk of severe COVID-19 and weakened immune response to COVID-19 vaccine as recommended by Centers for Disease Control and Prevention. The time to pneumonia development was defined as the time from symptom onset to the time when radiologic evidence of pneumonia was obtained. Viral clearance was defined as a Ct value > 30. COVID-19-related death was defined as 90-day death following imaging-confirmed pneumonia without any other plausible cause of death. A total of 467 patients with SARS-CoV-2 pneumonia were analyzed. Of these, 102 (22%) were moderately to severely immunocompromised. The median (IQR) time to pneumonia development was significantly longer in moderately to severely immunocompromised patients (9.5 [6–14] days) than the comparator (6 [3–8] days), p < 0.001), as was the median time to viral clearance (21 versus 12 days, p < 0.001). Moderately to severely immunocompromised status (aOR, 18.39; 95% CI, 5.80–58.30; p < 0.001) was independently associated with COVID-19-related death. Patients with moderately to severely immunocompromised conditions are likely to experience a more protracted course of SARS-CoV-2 pneumonia and a worse outcome than those without these conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has developed into a global pandemic since first reported from China in December 2019 [1]. The clinical spectrum of COVID-19 ranges from asymptomatic infection to critical illness [2]. Advanced age is the most important risk factor for severe COVID-19; others include obesity, diabetes mellitus, cardiovascular disease, chronic kidney disease, chronic lung disease, malignancy, and immunocompromised status [3, 4]. Of these others, immunocompromised status, including solid organ transplantation (SOT) and hematologic malignancy, is considered the highest risk factor for severe COVID-19 [4,5,6,7,8,9]. In addition, individuals with moderately to severely immunocompromised conditions due to specific medical conditions or from receipt of immunosuppressive medications or treatments, which result in increased risk of severe COVID-19 and poor response to COVID-19 vaccine, [10] may have the high risk of poor outcome of COVID-19.
Despite this, several studies have claimed that the increased risk is not a result from immunocompromised condition itself and that the outcomes of COVID-19 among immunocompromised individuals are very variable [11,12,13,14,15]. In addition, there is some suggestion that the reduced inflammatory response resulting from immunocompromised status is protective against severe COVID-19 [16,17,18]. However, data comparing the clinical characteristics and outcomes of COVID-19 in individuals with and without these immunocompromised conditions are limited, so it is still unclear how the weakened immune system contributes to the severity and outcomes of COVID-19. We have therefore compared the clinical characteristics and outcomes of patients with SARS-CoV-2 pneumonia who were considered as moderately to severely immunocompromised to those without these conditions.
Materials and methods
Study populations and design
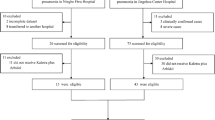
We retrospectively enrolled patients aged 18 years or more who had SARS-CoV-2 infection confirmed by polymerase-chain-reaction (PCR) in a 2,700-bed tertiary hospital, Seoul, South Korea, between February 2020 and April 2022. Clinical variables were collected from medical records, and we compared the patient characteristics, clinical course, and outcomes of patients with SARS-CoV-2 pneumonia who were moderately to severely immunocompromised with those who were not. The study was approved by the institutional review board of Asan Medical Center (IRB No 2022-0154).
Definitions
According to Centers for Disease Control and Prevention (CDC)’s recommendation, patients were considered as moderately to severely immunocompromised (IC) if they had medical conditions or treatments that resulted in increased risk of severe COVID-19 and weakened immune response to COVID-19 vaccine. Specifically, patients with hematologic malignancies or solid tumor on active treatment, SOT recipients taking immunosuppressive therapy, patients with moderate to severe primary immunodeficiency, or patients on active treatment with immunosuppressive or immunomodulatory agent were included [10]. SARS-CoV-2 pneumonia was defined if patients was confirmed as COVID-19 by PCR of nasopharyngeal specimens and had radiologic evidence of pneumonia on simple chest radiography or computed tomography. The time to pneumonia development was defined as the time from symptom onset to the first radiologic evidence of pneumonia. We repeated SARS-CoV-2 PCR during admission at least once per week, and viral clearance was defined as a cycle threshold (Ct) value > 30 from a respiratory specimen, because shedding of viable virus is rare in patients with Ct > 30 [19, 20]. The time to viral clearance was defined as the time from symptom onset to the first Ct exceeding 30. Causes of death were classified into COVID-19-related, other cause, indeterminate, or unclassifiable. All deaths following imaging-confirmed (chest X-ray or computed tomography) pneumonia without a plausible cause of death other than pneumonia were categorized as COVID-19-related death. All deaths with other identified causes were categorized as death due to other cause. Any case that did not fit any of the aforementioned criteria was categorized as indeterminate (e.g., the cause of death was unclear when SARS-CoV-2 pneumonia was accompanied by other factors, making it difficult to definitively attribute death to one cause or another) or unclassifiable (e.g., patients did not die in Asan Medical Center, and cause of death could not be evaluated).
In South Korea, the part of study period fell into the non-omicron-dominant era (before February 2022) and the omicron-dominant era (between February 2022 and April 2022). Since the SARS-CoV-2 variants might affect COVID-19 mortality, we stratified patients according to dominant circulating SARS-CoV-2 variants and performed a subgroup analysis of COVID-19-related death.
Statistical analysis
The Chi-square or Fisher’s exact test was used to analyze categorical variables, as appropriate. We used Student’s t test or the Mann–Whitney U test for continuous variables according to normality of the data. Logistic regression analysis was used to estimate the association between 90-day mortality due to SARS-CoV-2 pneumonia and other variables. Significant variables with p values < 0.10 in the univariate analysis, and other variables of clinical importance, were included in a multivariable analysis. In addition, a Cox proportional hazards model and survival curve adjusted for independent risk factors were used to compare survival rates in patients with and without IC conditions. To estimate rates of viral clearance, survival analysis was performed using Kaplan–Meier plots and the log-rank test. In addition, we create a Cox proportional hazards model and performed a multivariable analysis with significant variables with p values < 0.10 in the univariate analysis, and other variables of clinical importance to identify variables associated with viral clearance. All tests of significance were two-tailed, and p values < 0.05 were considered statistically significant. R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) was used for analysis and graphical presentation of the results.
Results
Study papulation
Of 621 hospitalized adult patients confirmed as COVID-19, 467 (75%) had radiologic evidence of pneumonia and were included in this study (Fig. S1). Their median age was 64 years, and 184 (39%) were female. Of the 467 patients, 102 (22%) were moderately to severely immunocompromised, and specific immunocompromised conditions are shown in Table 1 and S1. The remaining 365 (78%) patients were classified as the comparator group. The baseline clinical characteristics and outcomes of the two groups are shown in Tables 1 and 2. The IC patients were more likely than the comparators to have chronic kidney disease (46 [48%] vs 33 [9%]; p < 0.001), chronic lung disease (16 [16%] vs 17 [5%]; p < 0.001) and chronic liver disease (18 [18%] vs 20 [6%]; p < 0.001). However, members of the IC group were less likely to be obese (27 [27%] vs 158 [43%]; p = 0.003). Although most of the patients in the comparator group were infected by SARS-CoV-2 before the omicron-dominant era (322 [88%]), most of IC patients were infected during the omicron-dominant era (75 [74%]; p < 0.001) (Table 1). Patients with IC had less unvaccinated status (40%) than those without immunocompromised conditions (75%) at the time of SARS-CoV-2 infection (p < 0.001).
Comparison of clinical characteristics and viral clearance according to moderately to severely immunocompromised status
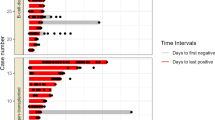
The median (IQR) time to pneumonia development was significantly longer in the IC group (9.5 [6,7,8,9,10,11,12,13,14] days) than in the comparator group (6 [3,4,5,6,7,8] days, p < 0.001) (Fig. 1). Cases of critical illness were less common among IC patients (33 [32%]) than in the comparator group (200 [55%]; p < 0.001). In addition, less patients in the IC group used a mechanical ventilator (19 [19%] vs 112 [31%]; p < 0.001) and be admitted to intensive care unit than the comparators (28 [28%] vs 182 [50%]; p < 0.001). There were no significant differences between the two groups in terms of COVID-19-specific treatments, except that tocilizumab was less often administrated to the IC group (21 [21%] vs 144 [40%]; p = 0.001) (Table 2).
Excluding 22 patients whose Ct value was not available, a total of 361 (81%) patients had Ct > 30 at some point during hospitalization, including 60 IC patients and 301 non-IC patients. There was a significant difference in median time to viral clearance between the two groups (21 versus 12 days, p < 0.001) (Fig. 2). In multivariable analysis, IC status (adjusted hazards ratio [aHR], 3.85; 95% confidence interval [CI], 2.50–5.88; p < 0.001) was independently associated with delayed viral clearance, and baricitinib treatment (aHR, 1.38; 95% CI, 1.02–1.89; p = 0.03) also was independently associated with delayed viral clearance (Table S2). However, remdesivir treatment (aHR, 0.69; 95% CI, 0.49–0.99; p = 0.046) was independently associated with shorter viral clearance.
The comparison of the proportion of patients with Ct value > 30 depending on moderately to severely immunocompromised status. Kaplan–Meier curve was shown, and the median time to viral clearance was indicated: 21 days for moderately to severely immunocompromised group and 12 days for the comparator group
Characteristics and variables associated with COVID-19-related death
Seventy-six (16%) patients died within 90 days of the date of SARS-CoV-2 pneumonia, and detailed causes of death are shown in Table 2. Of these patients, 54 (71%) died from SARS-CoV-2 pneumonia, and COVID-19-related deaths were significantly different in the IC group (24 [24%]) and the comparator group (52 [14%]; p = 0.046) (Table 2). Clinical characteristics according to COVID-19-related death are shown in Table 3. Patients who died from SARS-CoV-2 pneumonia were older (median [IQR] age, 76 [68–85] vs 62 [52–70] years, p < 0.001), and more likely to have cardiovascular disease (16 [30%] vs 57 [14%]; p = 0.005) than patients who did not.
Multivariate analysis revealed that age (adjusted odds ratio [aOR], 1.17; 95% CI, 1.12–1.22; p < 0.001) and IC status (aOR, 18.39; 95% CI, 5.80–58.30; p < 0.001) were independently associated with COVID-19-related death (Table 4). However, the omicron-dominant era (aOR, 0.33; 95% CI, 0.12-0.0.92; p = 0.03) was inversely associated with COVID-19-related death. After adjustment for age and dominant variant of the period, the survival rate in IC patients was significantly lower than that in the comparator group (p < 0.001) (Fig. 3).
Subgroup analysis for variables associated with COVID-19-related death
Patients who died due to SARS-CoV-2 pneumonia were more likely to be older in both the non-omicron-dominant era (median [IQR] age, 76 [70–85] vs 62 [53–70] years, p < 0.001) and the omicron-dominant era (median [IQR] age, 75 [66–85] vs 62 [52–69] years, p = 0.001) (Table S3). Multivariable analysis revealed that age was independently associated with COVID-19-related death in both the non-omicron-dominant era (aOR, 1.16; 95% CI, 1.11–1.22; p < 0.001) and the omicron-dominant era (aOR, 1.16; 95% CI, 1.06–1.28; p < 0.001). In addition, IC status was an independent risk factor for COVID-19-related death in both the non-omicron-dominant era (aOR, 15.22; 95% CI, 4.03–57.45; p < 0.001) and the omicron-dominant era (aOR, 10.84; 95% CI, 1.27–92.64; p = 0.03) (Table S4). Survival analyses stratified by dominant variant of the period are shown in Fig. S2.
Discussion
We have found that times to pneumonia development and viral clearance were significantly longer in the IC group than the comparator group. In addition, COVID-19-related mortality was significantly higher in IC patients than in the comparator patients, after adjustment for risk factors for COVID-19-related mortality. Therefore, our data provide important information for differential diagnosis of this protracted course of pneumonia and for de-isolation policy in IC patients, and emphasize the various interventions for these patients, including early antiviral treatment along with appropriate use of COVID-19-specific immunomodulators, as well as current COVID-19 vaccination policy.
Patients with SARS-CoV-2 infection are typically thought to progress to pneumonia over the course of a week from onset of symptoms [21], and our finding that the median time of progression to pneumonia from symptom onset in non-IC patients was 6 days is consistent with previous observations [21, 22]. However, the course of SARS-CoV-2 pneumonia was clearly more protracted in IC patients. Based on our understanding of the pathogenesis of COVID-19, we hypothesize that a weakened immune system may affect some characteristic of the cytokine storm and delay the development of pneumonia. Clinicians should therefore bear in mind the differential diagnosis of SARS-CoV-2 pneumonia in IC patients who have histories of SARS-CoV-2 infection several weeks previously.
The CDC recommends that the period of isolation of moderately to severely immunocompromised patients be extended to 20 days or more, in conjunction with a test-based strategy and consultation with an infectious diseases specialist [23]. However, there are limited data on viral shedding kinetics in IC patients with SARS-CoV-2 infection and these recommendations are largely based on studies involving small numbers of immunocompromised patients [24, 25]. Our study included a relatively large number of IC patients and showed that the median time to Ct > 30 in IC patients was 21 days compared with 12 days in non-IC patients in survival analysis. Hence, our findings support the current CDC’s recommendations that patients with severe COVID-19 isolate for at least 10 days and patients that are moderately to severely immunocompromised isolate for at least 20 days.
Although hematologic malignancies are the highest risk of progressing to COVID-19 [4,5,6,7,8,9], interestingly, Niemann et al. have reported that patients with chronic lymphocytic leukemia (CLL) have a reduced risk of death from COVID-19 in the omicron-dominant era [17]. They compared the outcomes of patients with CLL who were infected with SARS-CoV-2 before versus during the period of dominance of the omicron variant in Denmark, where the variant became dominant after December 17, 2021. They assigned the patients to one of four time periods and 30-day overall survival in the final period, from 1st January 2022 to 28th January 2022, being significantly higher than in the other three periods (p < 0.0077) [17]. However, our subgroup analysis stratified by the dominant era of variants revealed that IC status was identified as an independent risk factor for COVID-19-related death even in the omicron-dominant era. So, our findings emphasize the recent interventions recommended for these vulnerable patients, including early antiviral treatment with appropriate COVID-19-specific immunomodulator, and the current COVID-19 vaccination policy.
This study had some limitations. First, it was performed in a tertiary referral hospital, which makes it prone to possible selection and referral biases. In fact, patients in the comparator group did not reflect the general population, and most patients in this group had at least one risk factor for severe COVID-19 other than IC status. In addition, since IC patients also had other comorbidities associated with severity of COVID-19, the impact of IC status itself was difficult to assess. However, we compared mortality in the two groups after adjusting for other risk factors, and we were able to evaluate the effect of IC status itself on COVID-19-related death. Second, we defined IC status according to CDC`s recommendation. Since the immune system can be affected by the type of SOT, the cell of origin in hematologic malignancy, and use of certain chemotherapies and immunosuppressant drugs, there were significant differences in the level of immunosuppression among IC status and the fact that we combined heterogenous conditions and/or diseases as a one group could be a limitation of the study. Therefore, additional studies to clarify the level of immunosuppression (i.e., depending on COVID-19 vaccination response) and evaluate the impact of each IC condition separately on COVID-19 severity are needed. Third, the immunogenicity and effectiveness of COVID-19 vaccines are reported to be reduced in immunocompromised individuals [26] and could contribute to the outcomes of COVID-19 among patients in the IC group. In South Korea, COVID-19 vaccination campaign was conducted since March 2021 and 3-dose vaccination campaign was conducted since the end of December 2021. Therefore, patients who was infected SARS-CoV-2 before the campaign had never received COVID-19 vaccine and there was a discrepancy of vaccination rate in study population according to the period of SARS-CoV-2 infection. Although we performed multivariable analysis to offset the effect of this unbalanced vaccination status among the study population, further studies to identify association between vaccination status and viral clearance or COVID-19-related death in patients with IC status are needed. Finally, there were significant differences in terms of age, underlying diseases, the dominant era of variants, and COVID-19 vaccination status between patients with moderately or severely immunocompromised patients and the comparator. Although we tried to adjust these unbalanced variables by multivariable analyses, there might be still unmeasured confounders between two groups. Despite these limitations, our study is one of a few studies that included a large number of immunocompromised patients with COVID-19 and systemically evaluated the clinical characteristics, viral clearance, and outcomes in these vulnerable population.
In conclusion, there were longer delays from symptom onset to the development of pneumonia and viral clearance in moderately to severely immunocompromised patients than in patients without these conditions. In addition, moderately to severely immunocompromised status was an independent risk factor for COVID-19-related death and led to worse outcomes.
Change history
06 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10238-023-01016-1
References
Centers for Disease Control and Prevention. United States COVID-19 Cases, Deaths, and Laboratory Testing (NAATs) by State, Territory, and Jurisdiction. Available at: https://covid.cdc.gov/covid-data-tracker/?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcases-updates%2Fcases-in-us.html#cases_casesper100klast7days. Accessed Jun 28.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for disease control and prevention. JAMA. 2020;323(13):1239–42.
Centers for Disease Control and Prevention. COVID-19 (coronavirus disease): People with Certain Medical Conditions. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed Jun 28.
Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6.
Mehta V, Goel S, Kabarriti R, et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10(7):935–41.
Roberts MB, Izzy S, Tahir Z, Al Jarrah A, Fishman JA, El Khoury J. COVID-19 in solid organ transplant recipients: Dynamics of disease progression and inflammatory markers in ICU and non-ICU admitted patients. Transpl Infect Dis. 2020;22(5):e13407.
Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–8.
Lunski MJ, Burton J, Tawagi K, et al. Multivariate mortality analyses in COVID-19: Comparing patients with cancer and patients without cancer in Louisiana. Cancer. 2021;127(2):266–74.
Caillard S, Anglicheau D, Matignon M, et al. An initial report from the French SOT COVID registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98(6):1549–58.
Center of Disease Control and Prevention. People Who Are Immunocompromised. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-who-are-immunocompromised.html. Accessed November 23.
Molnar MZ, Bhalla A, Azhar A, et al. Outcomes of critically ill solid organ transplant patients with COVID-19 in the United States. Am J Transplant. 2020;20(11):3061–71.
Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021;105(6):1365–71.
Bossini N, Alberici F, Delbarba E, et al. Kidney transplant patients with SARS-CoV-2 infection: The Brescia Renal COVID task force experience. Am J Transplant. 2020;20(11):3019–29.
Avery RK, Chiang TP, Marr KA, et al. Inpatient COVID-19 outcomes in solid organ transplant recipients compared to non-solid organ transplant patients: A retrospective cohort. Am J Transplant. 2021;21(7):2498–508.
Kates OS, Haydel BM, Florman SS, et al. Coronavirus Disease 2019 in Solid Organ Transplant: A Multicenter Cohort Study. Clin Infect Dis. 2021;73(11):e4090–9.
Treon SP, Castillo JJ, Skarbnik AP, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135(21):1912–5.
Niemann CU, da Cunha-Bang C, Helleberg M, Ostrowski SR, Brieghel C. Patients with CLL have a lower risk of death from COVID-19 in the Omicron era. Blood. 2022;140(5):445–50.
Roeker LE, Eyre TA, Thompson MC, et al. COVID-19 in patients with CLL: improved survival outcomes and update on management strategies. Blood. 2021;138(18):1768–73.
Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 2020; 25(32).
Aranha C, Patel V, Bhor V, Gogoi D. Cycle threshold values in RT-PCR to determine dynamics of SARS-CoV-2 viral load: An approach to reduce the isolation period for COVID-19 patients. J Med Virol. 2021;93(12):6794–7.
Cohen PA, Hall LE, John JN, Rapoport AB. The early natural history of SARS-CoV-2 infection: Clinical observations from an urban, ambulatory COVID-19 clinic. Mayo Clin Proc. 2020;95(6):1124–6.
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506.
Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fif-you-are-sick%2Fquarantine-isolation.html. Accessed Aug 6.
Baang JH, Smith C, Mirabelli C, et al. prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J Infect Dis. 2021;223(1):23–7.
Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383(26):2586–8.
Lee A, Wong SY, Chai LYA, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632.
Funding
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), which is funded by the Ministry of Health & Welfare, Republic of Korea (Grant No. HW22C2045).
Author information
Authors and Affiliations
Contributions
Conceptualization: S-HK; Methodology: S-HK, JL; Data curation: JL, A RK, SWK; Software, formal analysis: JL; Writing-original draft: JL; Writing-review and editing: S-HK; Supervision: EC, SB, JJ, MJK, YPC, S-OL, SHC, and YSK; Funding Acquisition: S-HK.
Corresponding author
Ethics declarations
Conflicting interest
There are no conflicts of interest for any authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, J., Kim, A.R., Kang, S.W. et al. Protracted course of SARS-CoV-2 pneumonia in moderately to severely immunocompromised patients. Clin Exp Med 23, 2255–2264 (2023). https://doi.org/10.1007/s10238-022-00984-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00984-0