Abstract
We sought to explore the relationship between body mass index (BMI) and neurologic outcomes following acute COVID-19 infection. We conducted a retrospective electronic medical record-based cohort study enrolling adults with laboratory-confirmed acute COVID-19 infection who presented to 1 of 12 academic and community hospitals in Southwestern Ontario, Canada between April 1, 2020 and July 31, 2021. Primary subjective (anosmia, dysgeusia, and/or headache) and objective (aseptic meningitis, ataxia, delirium, encephalopathy, encephalitis, intracranial hemorrhage, ischemic stroke, and/or seizure) composite neurologic outcomes were assessed, comparing obese and overweight individuals to those with underweight/normal BMI indices, adjusting for baseline characteristics. Secondary outcomes (severity of illness, length of hospital stay, SARS-CoV-2 viral load, mortality) were similarly analyzed. A total of 1437 enrolled individuals, of whom 307 (21%), 456 (32%), and 674 (47%) were underweight/normal, overweight, and obese, respectively. On multivariable analysis, there was no association between BMI category and the composite outcome for subjective (odds ratio [OR] 1.17, 95% CI 0.84–1.64, Bonferroni p = 1.00 for obese; OR 1.02, 95% CI 0.70–1.48; Bonferroni p = 1.00 for overweight) and objective (OR 0.74, 95% CI 0.42–1.30, p = 0.29 for obese; OR = 0.80, 95% CI 0.45–1.43, p = 0.45 for overweight) neurologic manifestations. There was no association between BMI category and any secondary outcome measure and no evidence of effect modification by age or sex. This study demonstrates the absence of an association between BMI and neurologic manifestations following acute COVID-19 illness. Prospective studies using standardized data collection tools and direct measures of body fat are warranted to obtain more valid effect estimates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
COVID-19 is renowned for being one of the most morbid, deadly, and costly public health emergencies in human history. From the World Health Organization’s declaration of COVID-19 as a pandemic illness caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) to the time of writing, over 625 million infections, 6.5 million attributable deaths, and an incalculably large number of survivors with debilitating long-term post-infectious sequelae have been reported worldwide 1,2,3]. The pandemic has concomitantly touched the fabric of everyday life in all corners of the globe by way of its negative impacts on mental health, physical activity, social gatherings, education, and the economy 4,5,6]. Purported risk factors for severe COVID-19 illness and mortality include a constellation of chronic medical conditions, demographic characteristics such as older age and male sex, socioeconomic attributes including poverty, race, and ethnicity, and behavioral traits including cigarette smoking 7,8,9,10,11,12]. Obesity has recently been proposed as another potential risk factor for COVID-19 infection and severity of illness as well as being a repercussion of lockdowns and limited health promotion messaging during the pandemic. [8, 9, 8,9,13,14,15,16,17,18,19]
Supranormal body mass index is a major global public health threat and represents a pandemic of its own [20]. On a global scale, an estimated 650 million adults are deemed to be obese, and over 1.2 billion are deemed to be overweight [20]. Among adults, approximately 42% of Americans, 27% of Canadians, and 17% of Europeans are obese 21,22,23. Although obesity is considered a potential risk factor for a variety of bacterial and viral infections, controversy remains about a cause-and-effect relationship. [24]
Multinational ecologic studies have identified a correlation between obesity prevalence and COVID-19 incidence, and there appears to be an association between obesity and increased morbidity and mortality following COVID-19 infection in retrospective cohort studies 25,26,27,28]. Severity of illness in subjects with higher BMI is believed to be secondary to increased local and systemic inflammatory activity, impaired metabolic and immune system functioning, and barriers to care [29, 30].
Subjective neurologic manifestations, such as anosmia (decreased sense of smell), dysgeusia (abnormal sense of taste), and headache are common symptoms of acute COVID-19 infection, with limited post-hoc identification of a potential association between BMI and these outcomes. Objective neurologic manifestations, such as aseptic meningitis, ataxia, delirium, encephalitis, encephalopathy, intracranial hemorrhage, ischemic stroke, and seizures have also been reported following acute COVID-19, with one study documenting a higher risk of ischemic stroke in obese subjects 31,32,33,34,35,36].
Existing gaps in our current evidence base include uncertainty surrounding the potential neurotropism of SARS-CoV-2, the relationship between BMI and the development of neurologic manifestations following COVID-19 infection, whether increased COVID-19 severity among the obese is related to factors other than physiology (such as sociodemographics and systemic discrimination), and a lack of Canadian data on COVID-19 outcomes in obese and overweight subjects [37]. The aims of this study included determining the association between BMI and neurologic manifestations of acute COVID-19 infection, and the influence of sex and age on this relationship, as well enhancing our understanding of the relationship between BMI and SARS-CoV-2 viral load, length of hospital stay, severity of illness, and mortality following acute COVID-19 infection. We hypothesized that obesity would be associated with subjective and objective neurologic manifestations, with effect modification by age and sex. We also hypothesized that SARS-CoV-2 viral load, length of hospital stay, illness severity, and mortality would be higher in obese subjects.
Methods
Study design and population
This was a retrospective cohort study based on a review of electronic medical records at a network of 12 academic and community hospitals within six counties in Southwestern Ontario, Canada. Adults ≥ 18 years of age presenting to hospital with a PCR-confirmed first-onset, acute presentation of COVID-19 infection from April 1, 2020 to July 31, 2021 were included. The participating hospitals share a common electronic medical record (EMR) platform (Cerner PowerChart®, Cerner Inc., North Kansas City, Missouri) and rely on a single clinical laboratory for diagnostic microbiologic testing. Approval to conduct this study, with waiver of individual consent, was obtained from the Western University Research Ethics Board (London, Ontario, Canada) and the Harvard Longwood Campus Institutional Research Ethics Board (Boston, Massachusetts, USA).
Eligibility criteria
Inclusion criteria included residency in the province of Ontario, Canada, age ≥ 18 years, a positive SARS-CoV-2 real-time PCR test performed at the London Health Sciences Centre regional Microbiology Laboratory, and first presentation of acute COVID-19 infection at one of the participating hospitals during the study period. Exclusion criteria included transfer to or from a non-network hospital, hospital-acquired COVID-19 infection, any previous positive COVID-19 test performed at an external laboratory, prior receipt of a COVID-19 vaccine, receipt of blood or plasma product within the 60 day period prior to the onset of acute COVID-19 infection, and pregnancy. An electronic line listing of all positive COVID-19 real-time PCR results was generated to facilitate screening and enrollment of eligible individuals for the study.
Predictor variables
Our main predictor variable was BMI, defined as weight (kg)/height (m2), and was calculated from the most recently observed or self-reported height and weight in the EMR. Subjects were categorized as Obese (BMI > 30 kg/m2), Overweight (BMI > 25.0–29.9 kg/m2), and Underweight/Normal (BMI < 25.0 kg/m2) according to World Health Organization criteria. 21 Demographic variables included age (in years), sex, and race. Medical comorbidities including diabetes mellitus, hypertension, dyslipidemia, asthma, chronic obstructive pulmonary disease, obstructive sleep apnea, chronic kidney disease, chronic liver disease, coronary heart disease, congestive heart failure, and immunosuppression (T or B cell defects, HIV, hematologic malignancy, immunomodulatory drugs, chemotherapy, or radiotherapy in the preceding 6 months) were also recorded. Psychiatric conditions such as depression (and other mood disorders) and anxiety, and habits such as cigarette smoking (current, former, or rare/never), alcohol consumption (regular, occasional, or rare/never), and illicit substance use (any consumption or injection in the past 365 days) were ascertained. Median pre-tax household income in 2015 was used as a surrogate of socioeconomic status and was based on neighborhood-specific aggregate data from the 2016 Canadian Census as reported by Statistics Canada [38]. Given our a priori knowledge of the infrequent use of COVID-19 antiviral and immunomodulatory agents during the study period, we did not incorporate the use of therapeutic agents in our univariable or multivariable regression models.
Outcome measures
There were two primary endpoints in this study. The first was a composite of patient-reported neurologic symptoms including anosmia, dysgeusia, and/or headache. The second was a composite of clinically, radiologically, and/or biochemically observed neurologic conditions including aseptic meningitis, ataxia, delirium, encephalopathy, encephalitis, intracranial hemorrhage, ischemic stroke, and/or seizure. Secondary endpoints included hospital length of stay (in hours, scaled to number of days), severity of illness based on World Health Organization ordinal categories, SARS-CoV-2 PCR cycle threshold (an inverse surrogate of viral load), and all cause in-hospital mortality [39, 40]. Only outcomes that were deemed to have occurred as part of the acute illness/hospitalization were included in our analysis.
Data collection, sample size calculation, and handling of missing data
All study data was obtained directly from electronic medical records (including physician notes, nursing notes, diagnostic imaging reports) of eligible patients and from the regional microbiology laboratory. This information was subsequently entered into REDCap (Research Electronic Data Capture) web-based software (Vanderbilt University, Nashville, TN) housed on servers at the Lawson Research Institute of London Health Sciences Centre. After completing the data collection process, a Microsoft® Excel spreadsheet was generated and the data contained therein was imported into Stata® software version 16.1 (StataCorp, College Station, TX). We determined our estimated sample size requirement to be 948 participants, based on an expectation that each of the primary composite outcomes will occur in 5% of those in the Underweight/Normal (reference) BMI group and 10% of those in the Overweight and Obese BMI groups, along with a desired power of 80% and a two-sided significance level of 0.05. Missing data was handled using multiple imputation by chained equations (MICE) in Stata®.
Statistical analysis
Descriptive statistics included the number of subjects in each of the three BMI categories, with the Underweight/Normal BMI group serving as the reference category. Baseline characteristics were reported as means and standard deviations for continuous variables, and frequencies and proportions for categorical variables. Inferential statistical analyses included univariable and multivariable (adjusted for baseline characteristics) logistic regression for each of the two primary composite neurologic outcomes and for mortality, non-proportional odds logistic regression for severity of illness and length of hospital stay, and linear regression for SARS-CoV-2 cycle threshold. We conducted stratified analyses based on sex and dichotomous age (age < 65 years versus age ≥ 65 years) to assess for effect modification, and corresponding p values were obtained via interaction terms included in the regression model. For sensitivity analyses, we excluded subjects enrolled during the early waves of the pandemic (April 1, 2020–December 1, 2020) with December 1, 2021, representing the midpoint of our study’s timeframe. Effect measures were reported with their corresponding 95% confidence intervals and p-values. All p-values were adjusted for multiple testing using the Bonferroni method.
Results
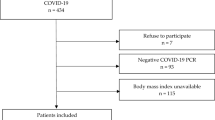
Figure 1 shows the geographic location of the 12 participating hospitals in Southwestern Ontario, Canada. A total of 1437 subjects met study eligibility criteria. Of these, 307 were in the Underweight/Normal BMI category, 456 were in the Overweight category, and 674 were in the Obese category. Baseline characteristics were fairly balanced with the exception of a relatively lower mean age in the Obese category, male predominance in the Overweight category, female predominance in the Obese category, and higher rates of diabetes mellitus, hypertension, and dyslipidemia in both the Overweight and Obese categories (Table 1). Baseline rates for smoking and alcohol consumption were mostly balanced among all categories although substance abuse in the past year was higher for the Underweight/Normal BMI group. Table 2 provides descriptive statistics for the subcomponents of each of the primary composite outcomes while Table 3 summarizes the results of univariable analyses for all outcomes.
Geographic location of the 12 participating hospitals in Southwestern Ontario, Canada (Adapted image reproduced under the Creative Commons Attribution-Share Alike 4.0 International license (https://commons.wikimedia.org/wiki/File:Canada_Southern_Ontario_location_map.png)
The primary composite outcome for subjective neurologic manifestations was realized in 37.1%, 37.7%, and 44.1% of Underweight/Normal, Overweight, and Obese subjects, respectively, with headache being the most common symptom. The primary composite outcome for objective neurologic manifestations was noted in 10.1%, 7.7%, and 5.9% of Underweight/Normal, Overweight, and Obese subjects, respectively, with delirium being the most common sign. When compared to the reference group, the unadjusted odds ratios for the subjective composite outcome were 1.02 (95% confidence interval (CI): 0.72, 1.45; Bonferroni p-value 1.00) and 1.34 (95% CI: 0.96, 1.88; Bonferroni p-value 0.348) for the Obese and Overweight groups, respectively (Table 2). Rates for each of the secondary outcomes appeared to be similar across exposure groups, except for mortality which was noted in 13.4%, 10.7%, and 8.8% of Underweight/Normal, Overweight, and Obese subjects, with all observations being non-statistically significant (Table 3).
The results of multivariable analysis for the two primary composite outcomes are shown in Fig. 2a, b as well as in Table 4. For the subjective composite outcome, the odds ratios for the Overweight and Obese categories compared to the Underweight/Normal category were statistically non-significant at 1.02 (95% CI: 0.70, 1.48; Bonferroni p-value = 1.00) and 1.17 (95% CI: 0.84, 1.64; Bonferroni p-value = 1.00), respectively. For the objective composite outcome, the respective odds ratios for the Overweight and Obese groups were statistically non-significant at 0.80 (95% CI: 0.45, 1.43; Bonferroni p-value = 1.00) and 0.74 (95% CI: 0.42, 1.30; Bonferroni p-value = 1.00).
Compared to Underweight/Normal BMI participants, being Overweight or Obese was not associated with three hospital length of stay categories, including hospitalization at or beyond 24 h, hospitalization for 7 days or longer, and hospitalization for 14 days or longer, with adjusted odds ratios of 0.98 (95% CI: 0.91, 1.05), 0.97 (95% CI: 0.91, 1.03), and 1.00 (95% CI: 0.95, 1.04), respectively, for the Overweight group, and 1.01 (95% CI: 0.95, 1.08), 0.99 (95% CI: 0.93, 1.05), and 1.00 (95% CI: 0.96, 1.05), respectively, for the Obese group, with all Bonferroni p-values = 1.00.
There was no significant relationship between being overweight or obese with severity of illness. The odds ratios for any inpatient hospitalization, hospitalization requiring high-flow oxygen or greater levels of respiratory support, and ICU admission were 0.86 (95% CI: 0.58, 1.28), 0.90 (95% CI: 0.60, 1.36), and 0.99 (95% CI: 0.52, 1.88), respectively, for the Overweight group, and 0.99 (95% CI: 0.58, 1.43), 1.07 (95% CI: 0.73, 1.57), and 1.52 (95% CI: 0.84, 2.80), respectively, for the Obese group, with all Bonferroni p-values = 1.00.
For SARS-CoV-2 cycle threshold, the adjusted odds ratios were non-significant at 1.14 (95% CI: 0.43, 3.01; Bonferroni p-value = 1.00) for the Overweight group and 1.45 (95% CI: 0.57, 3.69; Bonferroni p-value = 1.00) for the Obese group.
Mortality rates were non-significant, with adjusted odds ratios of 0.95 (95% CI: 0.92, 1.00; Bonferroni p-value = 1.00) and 0.97 (95% CI: 0.93, 1.01; Bonferroni p-value = 1.00) for the Overweight and Obese groups, respectively. Age and sex-stratified analyses for the two primary composite outcomes revealed no evidence of effect modification for the obese and overweight groups, respectively (Table 5). Sensitivity analysis revealed non-significant findings for the two primary composite outcomes when individuals who were enrolled prior to the study mid-point (December 1, 2020) were excluded from the analysis (Table 6).
Discussion
We demonstrated that obese and overweight study participants had no significantly increased risk of neurologic manifestations during acute COVID-19 illness based on our composite outcome measures. To our surprise, the point estimates for objective neurologic manifestations were lower for obese and overweight subjects, and vice versa for subjective neurologic manifestations. We also found no evidence of effect modification by age or sex in subgroup analyses for the two primary outcomes. All secondary outcome measures were not significantly different between obese and overweight subjects compared to underweight/normal subjects. Our sensitivity analysis involved restricting the focus to later waves of the pandemic as more information became available to guide the management of COVID-19 illness. However, the findings were not significantly different from those of the entire cohort.
Our results differ somewhat from those of other investigators. In a Chinese retrospective cohort study, Ye and colleagues observed that underweight subjects were significantly more likely to develop a headache following acute COVID-19 infection compared to other BMI groups [41]. Attia and colleagues in Egypt found a significantly higher incidence of altered mental status (delirium) post COVID-19 illness in subjects with a mean BMI of almost 34 kg/m2 compared to those with a mean BMI of approximately 31 kg/m2. [42] Ashrafi and colleagues conducted a cross-sectional study in a single tertiary hospital in the Middle East and found that headache, impaired taste, and reduced smell were the most common neurologic manifestations following COVID-19 infection [33]. However, these researchers modelled BMI as a continuous variable and found that individuals with neurologic manifestations had a significantly higher mean BMI compared to those without neurologic manifestations (26.64 kg/m2 versus 25.71 kg/m2, p = 0.02) [33]. In Italy, Vimercati and colleagues conducted a retrospective cohort study assessing chronic neurologic manifestations, which we did not assess [43]. However, these investigators observed that these neurologic signs were more frequent among those who were overweight and obese compared to those in lower BMI groups [43]. In the Philippines, Espiritu and coworkers conducted a retrospective cohort study on clinical outcomes following acute COVID-19 illness and found that obese persons with neurologic signs or symptoms had a lower rate of neurologic improvement at discharge compared to lower BMI groups [44]. In a recent review, Khan and coinvestigators from France argue that obesity is associated with a lower degree of perception of altered taste and smell, and that this lack of an alert to the onset of acute COVID-19 may result in poorer outcomes due to a delay in seeking care [45]. Table 7 summarizes the key findings of the aforementioned comparative studies. The findings of our study represent an important addition to the existing COVID-19 knowledge base. Our findings differ from those of other investigators although we acknowledge that limited comparative evidence exists. We believe that factors other than BMI may be playing a role in the development of neurologic symptoms following COVID-19. Thus, our study paves the way for the design of new experiments to investigate the relationship between BMI and a wide variety of infectious diseases, including but not limited to COVID-19. Our study is unique in developing a novel composite outcome measure for post-acute COVID-19 neurologic symptoms and signs among patients belonging to different BMI categories. No previous studies have assessed the relationship between BMI group and acute COVID-19 neurologic outcomes in any detail. We enrolled a diverse patient population from multiple regional hospitals and had around-the-clock on-site and remote access to detailed electronic medical record information, including physician and nurse-generated documents as well as diagnostic imaging reports, all of which fostered timely and accurate collection of clinical data. Our research endeavor faced a number of limitations, including a lack of information on race and ethnicity in the EMR, the a priori exclusion of vaccinated individuals due to the slow roll-out of COVID-19 vaccines in Canada, exclusion of antiviral or immunomodulatory therapy as a baseline predictor due to potential confounding by indication, an incomplete picture of baseline comorbidities due to variation in severity level between individual subjects, and the lack of follow-up post-hospital discharge. Information about baseline laboratory indices was not collected except for SARS-CoV-2 cycle threshold. The time period of our study did not extend beyond the initial hospital visit or admission, and we had a relatively small sample size for the outcomes being assessed. The most recent height and weight metrics in the EMR may not necessarily correspond to indices at the time of study inclusion. It also possible that milder neurologic symptoms may not have been documented in the EMR. Residual confounding is also likely to be present to some extent in observational studies. Furthermore, SARS-CoV-2 cycle threshold values may vary if obtained at different time points during the course of acute illness. Finally, BMI may not correspond to a well-defined intervention or exposure and may not provide an accurate measure of body fat.
Conclusions
Obese and overweight participants are not at increased risk of developing subjective or objective neurologic manifestations following COVID-19 infection, compared to those with an underweight/normal BMI. Future studies are warranted to explore the relationship between body fat composition and COVID-19-related neurologic outcomes using measures of adiposity other than, or in addition to, BMI, and to determine how vaccination status mediates the effects of obesity on acute COVID-19 infection. Studies designed to explore the relationship between degree of obesity (i.e., class I, II, and III obesity) and the development of neurologic manifestations following COVID-19 may provide further insight. A larger sample size using provincial/state and/or national health databases is recommended to increase study power. Furthermore, standardized electronic data collection forms for recording baseline characteristics, their severity, and all outcome measures should be considered for future COVID-19 prospective or retrospective cohort studies. Further studies will be needed to determine how BMI affects the prevalence and severity of post-acute-COVID syndrome, including neurologic manifestations such as cognitive dysfunction.
Data availability
The data that support the findings of this study are available from the REDCap database housed at the Lawson Research Institute, London Health Sciences Centre, London, Ontario, Canada. However, restrictions apply to the availability of these data under the existing ethics approval, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Lawson Research Institute.
Change history
09 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10238-022-00982-2
References
World Health Organization. WHO announces COVID-19 outbreak a pandemic. Regional Office for Europe. March 12, 2020. Available from https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic
Johns Hopkins University Coronavirus Resource Center. Accessed on October 24, 2022. Retrieved from https://coronavirus.jhu.edu/
World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Accessed on October 24, 2022. Retrieved from https://covid19.who.int/table
UNESCO. Learning losses from COVID-19 school closures could impoverish a whole generation. June 12, 2021. Retrieved from https://en.unesco.org/news/learning-losses-covid-19-school-closures-could-impoverish-whole-generation
International Monetary Fund. A Disrupted Global Recovery. IMFBlog. January 25, 2022. Retrieved from https://blogs.imf.org/2022/01/25/a-disrupted-global-recovery/
Wu T, Jia X, Shi H, Niu J, Yin X, Xie J. Prevalence of mental health problems during the COVID-19 pandemic: a systematic review and meta-analysis. J Affect Disord. 2021;281:91–8. https://doi.org/10.1016/j.jad.2020.11.117
Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21(1):855. https://doi.org/10.1186/s12879-021-06536-3
Booth A, Reed AB, Ponzo S, et al. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE. 2021;16(3):e0247461. https://doi.org/10.1371/journal.pone.0247461
Wolff D, Nee S, Hickey NS, Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2021;49(1):15–28. https://doi.org/10.1007/s15010-020-01509-1
Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021;76(2):428–55. https://doi.org/10.1111/all.14657
Raifman MA, Raifman JR. Disparities in the population at risk for severe illness from COVID-19 by race/ethnicity and income. Am J Prev Med. 2020;59(1):137–9. https://doi.org/10.1016/j.amepre.2020.04.003
Umnuaypomlert A, Kanchanasurakit S, Lucero-Prisno DE, Saokaew S. Smoking and risk of negative outcomes among COVID-19 patients: a systematic review and meta-analysis. Tob Induc Dis. 2021;19:09. https://doi.org/10.18332/tid/132411
Khan MAB, Smith JEM. “Covibesity”, a new pandemic. Obes Med. 2020;19:100282. https://doi.org/10.1016/j.obmed.2020.100282
Zhang X, Lewis AM, Moley JR, Brestoff JR. A systematic review and meta-analysis of obesity and COVID-19 outcomes. Sci Rep. 2021;11:7193. https://doi.org/10.1038/s41598-021-86694-1
Ullah W, Roomi S, Nadeem N, et al. Impact of body mass index on COVID-19-related in-hospital outcomes and mortality. J Clin Med Res. 2021;13(4):230–6. https://doi.org/10.14740/jocmr4239
Dana R, Bannay A, Bourst P, et al. Obesity and mortality in critically ill COVID-19 patients with respiratory failure. Int J Obes. 2021;10:1–10. https://doi.org/10.1038/s41366-021-00872-9
Katsoulis M, Pasea L, Lai AG, et al. Obesity during the COVID-19 pandemic: both cause of high risk and potential effect of lockdown? a population-based electronic health record study. Public Health. 2021;191:41–7. https://doi.org/10.1016/j.puhe.2020.12.003
Ekiz T, Pazarli AC. Relationship between COVID-19 and obesity. Diabetes Metab Syndr. 2020;14(5):761–3. https://doi.org/10.1016/j.dsx.2020.05.047
Jayawarden R, Jeyakumar TD, Misra A, Hills AP, Ranasinghe P. Obesity: a potential risk factor for infection and mortality in the current COVID-19 epidemic. Diabetes Metab Syndr. 2020;14(6):2199–203. https://doi.org/10.1016/j.dsx.2020.11.001
World Health Organization. Obesity and overweight. June 9, 2021. Retrieved from https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Centers for Disease Control and Prevention. Adult Obesity Facts. Accessed on October 6, 2022. Retrieved from https://www.cdc.gov/obesity/data/adult.html
Statistics Canada. Overweight and obese adults, 2018. Retrieved from https://www150.statcan.gc.ca/n1/en/pub/82-625-x/2019001/article/00005-eng.pdf?st=qMc_Yor5
Organization for Economic Co-operation and Development (OECD). Obesity among adults. Accessed October 6, 2022. Retrieved from https://www.oecd-ilibrary.org/sites/8cdeadfa-en/index.html?itemId=/content/component/8cdeadfa-en
Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes. 2013;37:33–340. https://doi.org/10.1038/ijo.2012.62
Kompaniyets L, Goodman AB, Belay B, et al. Body mass index and risk for COVID-19-related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death – United States, March–December 2022. Morb Mortal Wkly Rep. 2021;70(10):355–61. https://doi.org/10.15585/mmwr.mm7010e4
Kim T, Roslin M, Wang JJ, Kane J, Hirsch JS, Eun JK. Northwell Health COVID-19 Research Consortium. Body Mass Index as a Risk Factor for Clinical Outcomes in Patients Hospitalized with COVID-19 in New York. Obesity. 2021;2:279–84. https://doi.org/10.1002/oby.23076
Rossi AP, Gottin L, Donadello K, et al. Obesity as a risk factor for unfavourable outcomes in critically ill patients affected by Covid 19. Nutr Metab Cardiovasc Dis. 2021;31(3):762–8. https://doi.org/10.1016/j.numecd.2020.11.012
Suresh S, Siddiqui M, Abu Ghanimeh MA, et al. Association of obesity with illness severity in hospitalized patients with COVID-19: a retrospective cohort study. Obes Res Clin Pract. 2021;15(2):172–6. https://doi.org/10.1016/j.orcp.2021.02.006
Korakas E, Ikonomidis I, Kousathana F, et al. Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab. 2020;319(1):E105–9. https://doi.org/10.1152/ajpendo.00198.2020
Duong D. Link between obesity and COVID-19 may not be what it seems. CMAJNews. June 25, 2021. Retrieved from https://cmajnews.com/2021/06/25/covid-obesity-1095952/?msclkid=68132f92c25e11ecb268fc1efded2c0b
Johansson A, Mohamed MS, Moulin TC, Schloth HB. Neurological manifestations of COVID-19: a comprehensive literature review and discussion of mechanisms. J Neuroimmunol. 2021;358:577658. https://doi.org/10.1016/j.jneuroim.2021.577658
Bosque-Varela P, Moscote-Salazar LR. Obesity and stroke in the COVID19 era. Clin Neurol Neurosurg. 2020;196:105969. https://doi.org/10.1016/j.clineuro.2020.105969
Ashrafi F, Ommi D, Zali A, et al. Neurologic manifestations and their correlated factors in COVID-19 patients: a cross-sectional study. Arch Acad Emerg Med. 2021;9(1):e34. https://doi.org/10.22037/aaem.v9i1.1210
Whittaker A, Anson M, Harky A. Neurological Manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand. 2020;42(1):14–22. https://doi.org/10.1111/ane.13266
Vacaras V, Frunze S, Cordos AM. Neurological complications in COVID-19: A diagnostic challenge. J Med Life. 2021;14(2):216–24. https://doi.org/10.25122/jml-2021-0045
Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382(20):e60. https://doi.org/10.1056/NEJMc2009787
Bauer L, Kaksono BM, de Vrij FMS, Kushner SA, Harschnitz O, van Riel D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. 2022;45(5):P358–68. https://doi.org/10.1016/j.tins.2022.02.006
Statistics Canada. Census profile, 2016 Census. Available at https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/search-recherche/change-geo.cfm?Lang=E&Geo1=CD&Code1=3512&Geo2=POPC&Code2=1241&SearchType=Begins&SearchPR=01&B1=All&type=0
World Health Organization. R&D blueprint – novel Coronavirus – COVID-19 therapeutic trial synopsis. 2020. Accessed June 14, 2021. Retrieved from https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf
Morin F, Douillet D, Hamel JF, et al. Point-of-care ultrasonography for risk stratification of non-critical COVID-19 patients on admission (POCUSCO): a study protocol of an international study. BMJ Open. 2021;11(2):041118. https://doi.org/10.1136/bmjopen-2020-041118
Ye P, Pang R, Li L, Li HR, Liu SL, Zho L. Both underweight and obesity are associated with an increased risk of coronavirus disease (COVID-19) severity. Front Nutr. 2021;8:649422. https://doi.org/10.3389/fnut.2021.649422
Attia AS, Hussein M, Aboueisha MA, et al. Altered mental status is a predictor of poor outcomes in COVID-19: a cohort study. PLoS ONE. 2021;16(10):e0258095. https://doi.org/10.1371/journal.pone.0258095
Vimercati L, De Maria L, Quarato M, et al. Association between Long COVID and overweight/obesity. J Clin Med. 2021;10(18):4143. https://doi.org/10.3390/jcm10184143
Espiritu AI, Reyes NGD, Leochico CFD, et al. Body mass index and its association with COVID-19 clinical outcomes: Findings from the Philippine CORONA study. Clin Nutr. 2022;49:402–20. https://doi.org/10.1016/j.clnesp.2022.03.013
Khan AS, Hichami A, Khan NA. Obesity and COVID-19: Oro-Naso-sensory perception. J Clin Med. 2020;9(7):2158. https://doi.org/10.3390/jcm9072158
Acknowledgements
We thank Tung Pham, PhD Student, Department of Epidemiology, Harvard T.H Chan School of Public Health for providing statistical support related to multiple imputation. We also thank Dr. Heather Baer and Dr. Sara Oberg from the Department of Epidemiology, Harvard T.H. Chan School of Public Health for their feedback and mentorship during this study.
Funding
This authors received no specific funding to conduct this research.
Author information
Authors and Affiliations
Contributions
S.E. conceptualized the study. S.E. and W.H. developed the methodology. S.E. and D.O. ascertained clinical data from the Electronic Medical Record. A.C. extracted and reviewed all microbiologic data. R.D. reviewed all therapeutic data. S.E., W.H., and P.M.J. defined the statistical analysis plan. S.E. wrote the initial draft of the manuscript, with constructive feedback from all authors. All authors agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Approval to conduct this study, with waiver of individual consent, was obtained from the Western University Research Ethics Board (London, Ontario, Canada) and the Harvard Longwood Campus Institutional Research Ethics Board (Boston, Massachusetts, USA).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elsayed, S., Cabrera, A., Ouellette, D. et al. Association of body mass index with COVID-19-related neurologic sequelae: a retrospective cohort study. Clin Exp Med 23, 2239–2251 (2023). https://doi.org/10.1007/s10238-022-00965-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00965-3