Abstract
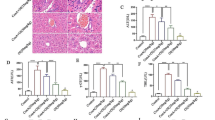
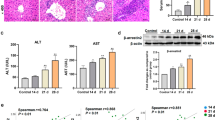
Activation of T cells and pro-inflammatory cytokines are essential for human autoimmune hepatitis. RAGE is one of the receptors for the inflammatory alarm molecule high mobility group box 1 (HMGB1), and it is involved in autoimmune hepatitis. However, the molecular mechanism of RAGE in the context of autoimmune hepatitis remains elusive. This study aimed to identify the function and mechanism of RAGE in autoimmune hepatitis. The role and underlying mechanisms of RAGE signaling-driven immune inflammatory response in ConA-induced experimental hepatitis were examined using the RAGE-deficient mice. We found that the RAGE deficiency protected the mouse from liver inflammatory injury caused by the ConA challenge. mRNA expression of VCAM-1, IL-6, and TNF-α within the livers is markedly decreased in RAGE-deficient mice compared to wild-type mice. In parallel, RAGE deficiency leads to reduced levels of the serum pro-inflammatory cytokines IL-6 and TNF-α as compared with wild-type control mice. RAGE-deficient mice exhibit increased hepatic NK cells and decreased CD4+ T cells compared with wild-type control mice. Notably, in vivo blockade of IL-6 in wild-type mice significantly protected mice from ConA-induced hepatic injury. Furthermore, RAGE deficiency impaired IL-6 production and was associated with decreased expression of Arid5a in liver tissues, a half-life IL-6 mRNA regulator. RAGE signaling is important in regulating the development of autoimmune hepatitis. Immune regulation of RAGE may represent a novel therapeutic strategy to prevent immune-mediated liver injury.








Similar content being viewed by others
Availability of data and materials
All data used during the current study available from the corresponding author on reasonable request.
Abbreviations
- RAGE:
-
The receptor for advanced glycation end-product
- AGEs:
-
Advanced glycation end products
- HMGB1:
-
High mobility group box 1
- AIH:
-
Autoimmune hepatitis patients
- EN-RAGE:
-
Extracellular newly identified receptor for advanced glycation end products binding protein
- sRAGE:
-
Soluble RAGE
- ConA:
-
Concanavalin A
- I/R:
-
Hepatic ischemia/reperfusion
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- MPO:
-
Myeloperoxidase
- ICAM-1:
-
Intercellular adhesion molecule-1
- VCAM-1:
-
Vascular cell adhesion molecule-1
- WT:
-
Wild-type mice
- Arid5a:
-
AT-rich interactive domain-containing protein 5a
References
Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–88.
Yatime L, Andersen GR. The specificity of DNA recognition by the RAGE receptor. J Exp Med. 2014;211(5):749–50.
Xie J, Méndez JD, Méndez-Valenzuela V, Aguilar-Hernández MM. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal. 2013;25(11):2185–97.
Jangde N, Ray R, Rai V. RAGE and its ligands: from pathogenesis to therapeutics. Crit Rev Biochem Mol Biol. 2020;55(6):555–75.
Gong Q, Zhang H, Li JH, et al. High-mobility group box 1 exacerbates concanavalin A-induced hepatic injury in mice. J Mol Med. 2010;88(12):1289–98.
Hua S, Ma M, Fei X, Zhang Y, Gong F, Fang M. Glycyrrhizin attenuates hepatic ischemia-reperfusion injury by suppressing HMGB1-dependent GSDMD-mediated kupffer cells pyroptosis. Int Immunopharmacol. 2019;68:145–55.
Ge X, Arriazu E, Magdaleno F, et al. High mobility group box-1 drives fibrosis progression signaling via the receptor for advanced glycation end products in mice. Hepatology. 2018;68(6):2380–404.
Jhun J, Lee S, Kim H, et al. HMGB1/RAGE induces IL-17 expression to exaggerate inflammation in peripheral blood cells of hepatitis B patients. J Transl Med. 2015;13:310.
Wu R, Liu Y, Yan R, Liu X, Duan L. Assessment of EN-RAGE, sRAGE and EN-RAGE/sRAGE as potential biomarkers in patients with autoimmune hepatitis. J Transl Med. 2020;18(1):384.
Yang L, Zhou L, Wang X, Wang W, Wang J. Inhibition of HMGB1 involved in the protective of salidroside on liver injury in diabetes mice. Int Immunopharmacol. 2020;89(Pt A):106987.
Zeng S, Feirt N, Goldstein M, et al. Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice. Hepatology. 2004;39(2):422–32.
Moy KA, Jiao L, Freedman ND, et al. Soluble receptor for advanced glycation end products and risk of liver cancer. Hepatology. 2013;57(6):2338–45.
Nyati KK, Masuda K, Zaman MM, et al. TLR4-induced NF-κB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. Nucleic Acids Res. 2017;45(5):2687–703.
Khambu B, Yan S, Huda N, Yin XM. Role of High-mobility group box-1 in liver pathogenesis. Int J Mol Sci. 2019;20(21):5314.
Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62.
Zhong H, Li X, Zhou S, et al. Interplay between RAGE and TLR4 Regulates HMGB1-induced inflammation by promoting cell surface expression of RAGE and TLR4. J Immunol. 2020;205(3):767–75.
Chen J, Duan L, Xiong A, et al. Blockade of IL-33 ameliorates Con A-induced hepatic injury by reducing NKT cell activation and IFN-γ production in mice. J Mol Med. 2012;90(12):1505–15.
Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208.
Xiang WQ, Feng WF, Ke W, Sun Z, Chen Z, Liu W. Hepatitis B virus X protein stimulates IL-6 expression in hepatocytes via a MyD88-dependent pathway. J Hepatol. 2011;54(1):26–33.
Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol. 2011;2011:345803.
Masuda K, Ripley B, Nishimura R, et al. Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proc Natl Acad Sci USA. 2013;110(23):9409–14.
Masuda K, Ripley B, Nyati KK, et al. Arid5a regulates naive CD4+ T cell fate through selective stabilization of Stat3 mRNA. J Exp Med. 2016;213(4):605–19.
Nyati KK, Agarwal RG, Sharma P, Kishimoto T. Arid5a regulation and the roles of Arid5a in the inflammatory response and disease. Front Immunol. 2019;10:2790.
Weinhage T, Wirth T, Schütz P, et al. The receptor for advanced glycation endproducts (RAGE) contributes to severe inflammatory liver lnjury in mice. Front Immunol. 2020;11:1157.
Acknowledgements
We thank Mr. Yong Xu and Zhihui Liang for technical assistance with flow cytometry.
Funding
This work was supported by grants from the National Natural Science Foundation of China (91542110, 81373167 to M. Fang).
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All animal protocols were approved by the Animal Research Committee at Tongji Medical College Animal Care and Use Committee (Animal permit number S2146).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Hua, S., Fang, D. et al. RAGE deficiency ameliorates autoimmune hepatitis involving inhibition of IL-6 production via suppressing protein Arid5a in mice. Clin Exp Med 23, 2167–2179 (2023). https://doi.org/10.1007/s10238-022-00960-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00960-8