Abstract
Vaccination represents the best strategy to fight COVID-19 pandemics, especially in immune compromised subjects. In chronic lymphatic leukemia patients, a marked impairment of the immune response to mRNA SARS-CoV-2 vaccine was observed. In this report, we analyzed anti-RBD and neutralizing antibodies in CLL patients after two doses of mRNA SARS CoV 2 vaccine and evaluated the impact of Bruton kinase inhibitory agents. Twenty-seven CLL patients vaccinated with mRNA vaccines against SARS CoV-2 were recruited. Serum IgG, IgM and IgA anti-RBD antibodies and neutralizing antibodies were detected, and antibody avidity was measured. Peripheral blood leukocytes subsets were evaluated by flow cytometry. After two vaccine doses anti-RBD IgG were produced in 11/27 (40.5%) of patients and levels of IgG and IgA anti RBD in CLL patients were sensibly lower than in controls. Neutralizing antibodies were detectable in 12/27 (44.5%) of the patients and their level was lower than that observed in controls. Disease burden and treatment with Bruton kinases inhibitors markedly impaired vaccine induced antibody response. However, in responder patients, antibody avidity was comparable to normal subjects, indicating that the process of clonal selection and affinity maturation takes place as expected. Taken together, these data confirm the impact of disease burden and therapy on production of anti-RBD and neutralizing antibodies and support the current policy of vaccinating CLL patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vaccination represents the best strategy to fight COVID-19 pandemics. Both DNA- and RNA-based vaccines have been approved and nowadays many million people have been vaccinated. Immune compromised subjects were not part of registration vaccine trials, but they obtained a high priority level in the access to vaccination because of their susceptibility to infections.
In chronic lymphocytic leukemia (CLL) patients, either the immune system highly dysregulated by the disease or the immune deficiency exacerbated by anti-leukemic treatment concur in inducing an impairment of immune responses and contribute to increase frequency and severity of infections and to reduce response to vaccines [1]. During treatment, ibrutinib significantly increases CD4+ and CD8+ T cells, especially of effector memory subset, and decreases the Treg/CD4+ T cell ratio. The immunomodulation exerted by ibrutinib is probably due to its off-target action, such as the inhibition of the IL-2 inducible kinase which is mainly expressed by T cells [2]. In acute myeloid leukemia, the BCL2 inhibitor venetoclax enhances T-cell effector function by increasing reactive oxygen species [3]. In CLL, it normalizes B, T, and NK-cell count, reduces the frequency of PD-1+ /CD8+ T cells, but also impairs the NK cells activation, reducing the anti-viral patient’s immunocompetence [4].
Previously, it has been reported that treatment naïve CLL patients respond poorly to HBsAg vaccination [5] or to pneumococcal vaccines, conjugated or not [6], with response rates between 20 and 40%. Immune response to hepatitis vaccine is nearly absent under treatment with BTK inhibitors. Recall responses to zoster vaccine are also reduced by therapy [5].
Several studies evaluated the response of CLL patients to mRNA SARS CoV 2 vaccines, measuring serum anti-spike antibodies after one [7], two [7,8,9] or three [10] doses. A marked impairment of the immune response was observed, with a response rate of 40–75%. Recently, in a cohort of 286 patients, spike-specific antibody responses were observed in 34% after one and in 75% after two vaccines compared to 94% in healthy donors, especially in cases receiving BTK inhibitors and with low IgA levels [7]. In another series, only 23% of CLL treated patients had detectable antibodies versus 70% of untreated subjects [11]. Among the factors influencing antibody production, in addition to the ongoing therapy also timing of antibody evaluation may account for inter-studies differences.
The spike (S) protein is a complex antigen, and it is conceivable that only part of induced antibodies reacts with the portion of the receptor binding domain (RBD) that interacts with ACE 2 receptor and mediates viral entry into the cells. Thus, evaluation of antibodies that block RBD interaction with ACE 2 represents a better tool to infer protection from COVID-19. So far, only two studies evaluated neutralizing antibodies (NAbs) induced by vaccination in CLL patients; in the first one, the median NAbs inhibition titer was 17% for patients with CLL, Waldenstrom Macroglobulinemia or other non-Hodgkin’s lymphomas versus 32% in controls [12]. In the second study, 160 cancer patients with CLL or other solid tumors exhibited reduced NAbs, especially CLL patients that presented values below the detection limit in 50–60% of the cases [13].
In this report, we analyzed anti-RBD and neutralizing antibodies in CLL patients comparing them with the immune responses observed in healthy individuals after two doses of mRNA SARS CoV 2 vaccine.
Material and methods
Patients and methods
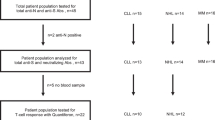
Twenty-seven CLL patients regularly followed at the Hematology Unit of Pisa University Hospital and vaccinated with mRNA vaccines (Comirnaty—BNT162b2, n = 21; SpikeVax mRNA-1273, n = 6) against SARS CoV-2 were recruited into the study.
At the time of enrollment, the following clinical data were collected for each patient: age, gender, stage of disease, treatment status, laboratory parameters and presence of hypogammaglobulinemia (Table 1). Analyses of genomic aberrations by fluorescent in situ hybridization (FISH) and mutational status of the immunoglobulin heavy chain variable (IGHV) gene have been also included.
Twenty-one health care workers (HCW), vaccinated with mRNA BNT162b2, served as control group (mean age ± SD = 46.8 ± 12.9; M/F = 5/16).
Whole blood was collected before the first dose (T0) and 21 days after the second one (T2). Sera were collected and kept frozen at − 60 °C until use.
No patient nor any control previously contracted SARS-CoV-2 infection before recruitment for the study.
The study was approved by the local Ethical Committee (Approval N° 17522) and patients signed an informed consent the day of enrollment.
FACS analysis of peripheral blood
Peripheral blood granulocytes, monocytes, T and B lymphocytes, NK cells and CD4+ and CD8+ T naïve, central memory (CM) and terminally differentiated effector memory (TEMRA) subsets were evaluated by flow cytometry in 18 out of 27 patients.
The panel used in this study is a mix of anti-CD antibodies (CD4, CD5, CD3, CD19, CD56, CD45, CD8, CD45RA, CCR7, CD27—BD Biosciences, See Table I). We incubated blood samples (100 μl) with the antibody mix for 15 min; erythrolysis was performed with FACS Lysing solution (2 ml, 10 min). Samples were then washed with phosphate buffer saline (PBS) and centrifuged (1000 g, 10 min). Cells were resuspended in PBS and flow cytometric analyses were performed with a BD FACSCanto II flow cytometer. For each sample 100.000 events were analyzed.
Anti-RBD antibody titers
Antibodies were measured by solid phase assay, on plates coated with recombinant Receptor Binding Domain (SARS-CoV-2 Spike protein aa319–541), as previously described [14]. IgG, IgM and IgA anti-RBD antibodies were detected.
Analysis of neutralizing antibodies
To detect neutralizing antibodies, the kit SPIA (Spike Protein Inhibition Assay, DiaMetra, Perugia, Italy) was employed according to manufacturer’s instructions. In this assay, patient’s antibodies compete with peroxidase-conjugated ACE2 for the binding to viral RBD coated on the solid phase.
Inhibition value was calculated using this formula:
Avidity assay
Antibody avidity was evaluated in a subgroup of 8 patients, by means of an Avidity ELISA, employing different concentrations of urea as chaotropic reagent. The Avidity Index (AI) was calculated as the extrapolated urea concentration that displaces 50% of serum binding with respect to the control wells using the approach described by Polanec et al. [15]. The area under the curve (AUC) derived by plotting on the y-axis the % binding with respect to the control wells and on x-axis the different Urea molar concentrations was employed to compare the avidity of anti-RBD in vaccinated CLL patients versus vaccinated healthy care workers (HCW).
Statistical analysis
Statistical analysis was performed using IBM-SPSS® Statistics, and GraphPad Prism statistical packages. Antibody levels at different time points were compared by Kruskall-Wallis. Results of anti-RBD Ig were expressed as Odds Ratio (OR) of a positive internal control set at 1,0. Cut off values have been set at the 97.5th percentile of the healthy care workers (HCW) evaluated before vaccination. P < 0.05 was considered as significative.
Results
Demographic data, disease characteristic and ongoing or previous therapies administered to the 27 enrolled patients are summarized in Table 1. Median age was 70.6 years, with a prevalence of males (66.7%). Binet disease stage was A in 40% and B in 60% of cases. Predominant IgHV mutational status was unmutated (84.6%). Only one patient showed TP53 mutation. About treatment status, 37% of patients were treatment naïve, 48.1% were on therapy and 14.9% were off therapy after achieving clinical remission; 46.2% were on ibrutinib and 30.8% on rituximab plus venetoclax; one patient was on idelalisib.
Anti-RBD antibodies in vaccinated patients
After two vaccine doses anti-RBD IgG were produced in 11/27 (40.5%) of patients while IgM and IgA were induced in 4/27 (14.8%) and 5/27 (18.5%), respectively. Levels of IgG and IgA anti RBD in CLL patients were sensibly lower than in HCW used as control (p < 0.0001) (Fig. 1A–B, C–D, E–F and Supplementary Figure 1).
Anti-RBD and neutralizing antibodies in LLC patients. Distribution of IgG (Fig. 1A), IgM (1C) and IgA (1E) anti-RBD and neutralizing antibodies (1G) induced by mRNA vaccine in LLC patients as compared with health care workers (HCW). Levels of IgG (Fig. 1B), IgM (1D) and IgA (1F) anti-RBD and neutralizing antibodies (1H) before the first (T0) and after the second (T2) dose of mRNA vaccine. Results of anti-RBD are represented as odds ratio of a positive internal control (OR). Results of neutralizing antibodies as percentage of inhibition of the binding of ACE to RBD. p < 0.05 was considered as significant
As far as the production of NAbs is concerned, antibodies inhibiting the interaction of RBD with ACE2 were detectable in 12/27 (44.5%) of the patients, compared with 100% of healthy controls (p < 0.0001), and their level was lower than that observed in controls (p < 0.0001). (Fig. 1G–H).
Regarding potential factors influencing vaccine efficacy in CLL patients, we compared the amount of anti-RBD antibodies and NAbs in treated vs untreated patients. Anti-RBD and NAbs were significantly higher in untreated patients (p < 0.01 and p < 0.01, respectively).
Conversely, in the subgroup of the 12 patients who developed anti-RBD antibodies after 2 vaccination doses only 3 were under treatment, while 9 were therapy-naïve or far from the last treatment.
Among subjects under therapy, only one out of 7 patients (14.3%) receiving ibrutinib produced low amounts of NAbs and the levels of anti-RBD IgG and NAbs were much lower than those measured in controls (p < 0.005) (Fig. 2). Out of the 4 patients under venetoclax treatment, only one developed high titers of anti RBD and NAbs.
Anti RBD Ab, SPIA and Ibrutinib. Levels of IgG, IgM and IgA anti-RBD (Fig. 2A) and neutralizing antibodies (2B) in LLC patients untreated as compared with patients treated with Ibrutinib. Results of anti-RBD are represented as odds ratio of a positive internal control (OR). Results of neutralizing antibodies as percentage of inhibition of the binding of ACE to RBD. p < 0.05 was considered as significant
The titer of Neutr Ab antibodies was inversely correlated with beta-2 microglobulin levels (p < 0.005), major lymph node dimension (p < 0.005), spleen size (p < 0.05) and with affected lymph nodes area (p < 0.005) (Supplementary Fig. 2).
The time elapsed between diagnosis and treatment start or between the last therapy and vaccination did not impact on the antibody production.
Antibody avidity was evaluated in a subgroup of 8 patients under treatment by means of chaotropic ELISA: mean RBD Avidity Index (AI) was 6.79 ± 1.79 (AUC = 605.9), not statistically different from that obtained for the vaccinated controls (AUC = 586.9) (Fig. 3).
Anti-RBD antibody Avidity in vaccinated LCC and HCW. Antibody avidity was measured by avidity ELISA using different urea concentrations. Curves of binding to RBD obtained with sera from vaccinated CLL patients (●) and with sera from vaccinated health care workers (□) are shown. Results are expressed as the area under the curve (AUC) derived by plotting on the y-axis the % of binding and on x-axis the different Urea molar concentrations
We also analyzed the correlation between vaccine induced anti-RBD or NAbs and peripheral blood cell subset.
We evaluated by flow cytometry the total number of granulocytes, monocytes and lymphocytes (identifying cells by side scatter and CD45 staining); number of T (CD3+), B (CD19+ , CD20+) and NK (CD3−, CD56+) cells; number of CD4+ and CD8+ T naïve (CD27− /CCR7− and CD45RA+), central memory (CD27 + /CCR7 + and CD45RA-) and terminally differentiated effector memory (CD27+ /CCR7+ and CD45RA+) cells.
The levels of NAbs were inversely correlated with baseline WBC number (p < 0.05), total lymphocytes (p < 0.05) and B lymphocytes (p < 0.05).
On the contrary, CD4+ and CD8+ T memory or naïve cell number did not significantly affect the immune response induced by mRNA vaccination.
Discussion
Our study, even if conducted in a small cohort, shows that CLL patients poorly respond to SARS CoV2 mRNA vaccines: indeed, anti-RBD antibodies and NAbs are produced only in 40% of vaccinated patients and at lower levels than in vaccinated healthy subjects. These results are comparable to those previously published [8,9,10, 16]. On the contrary, Parry et al. [7] described low titers of anti-spike antibodies in 75% of the patients after the second dose; clinical features of this cohort, and namely the higher proportion of treatment-naïve patients, may explain this discordance.
The production of NAbs has also been evaluated in other studies that report positive results in 40 to 50% of the patients [12] [13, 17], analogously to our series.
NAbs have been evaluated by means of an assay based on the inhibition of RBD-ACE2 interaction. Plaque reduction neutralization tests represent the golden standard for the detection of neutralizing antibodies. However, a positive correlation between neutralization assays and inhibition of RBD-ACE2 interaction has been obtained by many authors and the antibody-mediated blockage of ACE2-spike interaction has been considered a SARS-CoV-2 surrogate virus neutralization test [18].
Interestingly, notwithstanding the low percentage of serological immune response, we demonstrated that in responder patients, antibody avidity was comparable to normal subjects, indicating that the process of clonal selection and affinity maturation takes place as expected. This observation is relevant, because it sustains once again the need of proceeding with vaccination also in CLL patients.
The possibility that the use of different vaccines may partially improve the immune response of CLL patients cannot be completely ruled out. A lower titer of antibodies has been detected in patients vaccinated with BNT162b2 vs mRNA-1273 [16]. In our study, most patients were vaccinated with BNT162b2 only a few patients were vaccinated with mRNA-1273 vaccine and their antibody titer was not different.
As shown in the present study, disease burden, ongoing and past therapies are the main factors affecting antibody responses. As previously reported, in the present report we observed lower antibody levels in patients treated with ibrutinib; because of the low number of patients receiving other treatments, data on venetoclax or chlorambucil need further investigation.
BTK inhibitors, irreversibly inactivating BTK, interfere with BCR signaling and thus with B cell development, proliferation, differentiation and activation. Thus, it is conceivable that a therapy directly targeting B cells may impair antibody responses to novel antigens. However, reducing the number of exhausted T cells and immunosuppressive Treg, BTK inhibitors may restore a normal T cell compartment and also favor dendritic cell maturation, thus positively affecting immune responses [19].
In addition to B cells, also T cell response is essential to protect against Coronavirus: specific IFN-y and IL-2-mediated immune responses were observed in CLL patients irrespective of anti-S production or neutralizing activity [20]. Even if we did not evaluate anti-SARS CoV 2 T responses, the phenotype of peripheral T cells in our patients is compatible with a normal T response. Cellular immunity to spike should be further evaluated in CLL vaccinated subjects to get more insights on the level of protection that can be achieved by vaccination.
Herishanu [21] recently reported a case series of persistence of SARS-CoV-2 antibodies after the second BNT162b2 mRNA COVID-19 vaccine dose in CLL patients. At 6 months after the second vaccine dose antibody titers were lower in patients compared with controls, but still detectable, and patients on active treatment have the lowest titers.
In a series of 536 Italian patients with different hematological malignancies 37% died; if compared with chronic myeloid malignancies, the probability of death for CLL patients was 60% higher [22]. In another CLL cohort, where 79% of the patients had been hospitalized for COVID-19, CLL disease burden significantly impacted on outcome: milder disease was observed in untreated patients, age did not impact on mortality and BTK inhibitors appeared to exert a protective effect, as observed in the CLL patients we studied [23].
A restoration of cellular and humoral immune function can be induced also by target therapy and this ability, coupled with the anti-inflammatory action played by some inhibitors such as ibrutinib, explains why continuing these therapies during moderate COVID-19 infection could be useful.
The recent approval by EMA of tixagevimab and cilgavimab (website: https://www.ema.europa.eu/en/medicines/human/EPAR/evusheld) offers new opportunities to non-responder patients. These antibodies have been designed to attach to the spike protein at two different sites, avoiding the virus to enter the cells, to multiply and cause COVID-19. The combination drug is indicated for prophylaxis of COVID-19 in adults and adolescents with active hematological malignancies, those who received allogeneic transplantation, in active treatment with high-dose corticosteroids, alkylating agents, antimetabolites, transplant-related immunosuppressive drugs, cancer chemotherapeutic agents, including B-cell–depleting agents in CLL.
Taken together, all available data support the current policy of vaccinating CLL patients, irrespective of the ongoing therapy and suggest that measurement of anti-RBD and neutralizing antibodies might help in planning passive immunotherapy with anti-Spike antibodies.
References
Griggio V, Perutelli F, Salvetti C, et al. Immune dysfunctions and immune-based therapeutic interventions in chronic lymphocytic leukemia. Front Immunol. 2020;11: 594556. https://doi.org/10.3389/fimmu.2020.594556.
Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127:3052–64. https://doi.org/10.1172/JCI89756.
Lee JB, Khan DH, Hurren R, et al. Venetoclax enhances T cell-mediated antileukemic activity by increasing ROS production. Blood. 2021;138:234–45. https://doi.org/10.1182/blood.2020009081.
Kohlhapp FJ, Haribhai D, Mathew R, et al. venetoclax increases intratumoral effector T cells and antitumor efficacy in combination with immune checkpoint blockade. Cancer Discov. 2021;11:68–79. https://doi.org/10.1158/2159-8290.CD-19-0759.
Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137:185–9. https://doi.org/10.1182/blood.2020008758.
Hartkamp A, Mulder AHL, Rijkers GT, et al. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine. 2001;19:1671–7. https://doi.org/10.1016/S0264-410X(00)00409-6.
Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11:136. https://doi.org/10.1038/s41408-021-00528-x.
Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–73. https://doi.org/10.1182/blood.2021011568.
Roeker LE, Knorr DA, Thompson MC, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35:2703–5. https://doi.org/10.1038/s41375-021-01270-w.
Marlet J, Gatault P, Maakaroun Z, et al. Antibody responses after a third dose of COVID-19 vaccine in kidney transplant recipients and patients treated for chronic lymphocytic leukemia. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9101055.
Agha M, Blake M, Chilleo C, et al. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. medRxiv. 2021. https://doi.org/10.1101/2021.04.06.21254949.
Gavriatopoulou M, Terpos E, Kastritis E, et al. Low neutralizing antibody responses in WM, CLL and NHL patients after the first dose of the BNT162b2 and AZD1222 vaccine. Clin Exp Med. 2021. https://doi.org/10.1007/s10238-021-00746-4.
Zeng C, Evans JP, Reisinger S, et al. Impaired neutralizing antibody response to COVID-19 mRNA vaccines in cancer patients. Cell Biosci. 2021;11:197. https://doi.org/10.1186/s13578-021-00713-2.
Pratesi F, Caruso T, Testa D, et al. Bnt162b2 mRNA SARS-CoV-2 vaccine elicits high avidity and neutralizing antibodies in healthcare workers. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060672.
Polanec J, Seppälä I, Rousseau S, Hedman K. Evaluation of protein-denaturing immunoassays for avidity of immunoglobulin G to rubella virus. J Clin Lab Anal. 1994;8:16–21. https://doi.org/10.1002/JCLA.1860080105.
Chung DJ, Shah GL, Devlin SM, et al. Disease- and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies. Blood Cancer Discov. 2021;2:568–76. https://doi.org/10.1158/2643-3230.BCD-21-0139.
Terpos E, Gavriatopoulou M, Fotiou D, et al. Poor neutralizing antibody responses in 132 patients with CLL, NHL and HL after vaccination against SARS-CoV-2: a prospective study. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13174480.
Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein-protein interaction. Nat Biotechnol. 2020;38:1073–8. https://doi.org/10.1038/s41587-020-0631-z.
Zhu S, Gokhale S, Jung J, et al. Multifaceted Immunomodulatory Effects of the BTK Inhibitors Ibrutinib and Acalabrutinib on Different Immune Cell Subsets - Beyond B Lymphocytes. Front Cell Dev Biol. 2021. https://doi.org/10.3389/FCELL.2021.727531.
Shen Y, Freeman JA, Holland J, et al. COVID-19 vaccine failure in chronic lymphocytic leukaemia and monoclonal B-lymphocytosis; humoural and cellular immunity. Br J Haematol. 2022;197:41–51. https://doi.org/10.1111/bjh.18014.
Herishanu Y, Avivi I, Levi S, et al. Six-month antibody persistence after BNT162b2 mRNA COVID-19 vaccination in patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:148–51. https://doi.org/10.1182/bloodadvances.2021005998.
Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737–45. https://doi.org/10.1016/S2352-3026(20)30251-9.
Scarfò L, Chatzikonstantinou T, Rigolin GM, et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34:2354–63. https://doi.org/10.1038/s41375-020-0959-x.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. The work was funded by Italian Ministry of Health grant COVID-2020-12371849.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. CB and FM were in charge for patient’s recruitment and care. TC and GM and FP planned and performed the serological analysis. PS and VG planned and performed the cytofluorimetric analysis. The first draft of the manuscript was written by CB, SG and PM and all authors commented on previous version of the manuscript. PM and SG supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Local Ethics Committee (Approval N° 17522). Written informed consent was obtained by participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baratè, C., Caruso, T., Mavilia, F. et al. Induction of neutralizing antibodies in CLL patients after SARS-CoV-2 mRNA vaccination: a monocentric experience. Clin Exp Med 23, 1197–1203 (2023). https://doi.org/10.1007/s10238-022-00877-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00877-2