Abstract
The role of platelet function indices—platelet count (PLT), mean platelet volume (MPV), platelet distribution width (PDW), plateletcrit (PCT), immature platelet fraction (IPF), and platelet mass index (PMI)—in psoriasis is uncertain. This systematic review and meta-analysis aimed to evaluate the association of these platelet biomarkers with both presence and severity of psoriasis. We searched MEDLINE (Ovid), Embase (Ovid), and the Cochrane Library from inception to November 2021. To evaluate the association of platelet function indices and psoriasis, we recorded mean differences (MD) and 95% confidence intervals (CI) as well as correlation coefficients (r) for each included study, and generated summary estimates using random-effects inverse-variance modelling. We screened 1,079 unique studies, and included 33 studies with 6724 patients in the quantitative analyses. Compared with controls, patients with psoriasis had higher PLT (MD 12.86 × 109/L, 95% CI 6.34–19.39, p < 0.001), MPV (MD 0.61fL, 95% CI 0.31–0.92, p < 0.001), and PCT (MD 0.05%, 95% CI 0.01–0.09, p = 0.010), but similar PDW (MD 0.16%, 95% CI -0.46–0.79, p = 0.610). Psoriasis Area and Severity Index (PASI) was weakly correlated with PLT (r 0.17, 95% CI 0.06–0.28, p = 0.003), MPV (r 0.36, 95% CI 0.22–0.49, p < 0.001), and PDW (r 0.17, 95% CI 0.08–0.26, p < 0.001). Study numbers were insufficient to judge the relationship of IPF and PMI with psoriasis presence, or PCT, IPF, and PMI with psoriasis severity. In summary, PLT, MPV, and PCT are significantly elevated in patients with psoriasis, and PLT, MPV, and PDW are weakly correlated with PASI. Future studies are needed to evaluate the independent diagnostic and prognostic potentials of these biomarkers in patients with psoriasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriasis is a common chronic inflammatory skin disorder with a variable worldwide prevalence of up to 11.4% in adults and 1.4% in children [1]. Although classically associated with the development of multiple inflammatory skin plaques in an extensor distribution, a growing body of evidence now substantiates an appreciation of psoriasis as a systemic disease with associated extracutaneous comorbidities [2]. In particular, patients with psoriasis are more likely to exhibit comorbid atherosclerotic vascular disease—cardiovascular, cerebrovascular, and peripheral vascular—and its risk factors such as obesity, metabolic syndrome, hypertension, diabetes, and smoking [3, 4].
The underlying pathogenesis of psoriasis and its relationship to cardiovascular disease remains incompletely understood. The association between platelet activation and cardiovascular disease is known [5,6,7]. More recently, studies have investigated the association between platelet activation and psoriasis [8, 9], reflecting an emerging sphere of research which aims to investigate the diagnostic and prognostic potentials of simple blood parameters in the context of increasing access to, cost-effectiveness, and routinisation of haematological assays [10,11,12,13,14,15]. Specifically, platelet count (PLT), mean platelet volume (MPV), platelet distribution width (PDW), plateletcrit (PCT), immature platelet fraction (IPF), and platelet mass index (PMI) are haematological markers of platelet function which have demonstrated associations with both cardiovascular-related diseases and skin diseases [16,17,18,19]; however, their significance in psoriasis is uncertain.
Understanding how abnormal platelet structure and function relate to the presence and severity of psoriasis may refine our understanding of disease pathophysiology and contribute to new insights in risk stratification and prognosis. We therefore performed a systematic review and meta-analysis to determine the association of platelet indices with psoriasis.
Patients and methods
Study design and registration
This systematic review and meta-analysis evaluated study-level data, and was reported in compliance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [20] and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) [21] statements. We prospectively registered review protocol details with PROSPERO (CRD42021290920); there were no protocol deviations.
Criteria for considering studies for this review
We included all original research studies which reported platelet parameters of PLT, MPV, PDW, PCT, IPF, and PMI grouped by patients with and without psoriasis. We defined PLT as the count of platelets as measured in × 109/L, MPV as the average size of individual platelets measured in fL, PDW as the variability in platelet size measured in %, PCT as plateletcrit and a reflection of the mass of platelets measured in %, IPF as the fraction of immature platelets in blood and a marker of thrombopoietic activity measured in %, and PMI as the product of PLT and MPV and a reflection of the plaque forming capacity of platelets. We excluded non-human studies, conference abstracts and presentations, case reports and series, expert opinions, and letters to the editor.
Search methods for identification of studies
We searched MEDLINE (Ovid), Embase (Ovid), and the Cochrane Library from inception to November 2021, and employed a comprehensive set of search terms for PLT, MPV, PDW, PCT, IPF, PMI, and psoriasis in our search strategy (Online Resource 1). We placed no limits on language or publication period. We subsequently reviewed the reference and citation lists of included studies for further potentially relevant studies. We used the online software ‘Covidence’ [22] to organise the search process.
Study selection
Two review authors (ZL and LAP) independently screened the titles and abstracts of each search result against eligibility criteria for relevant studies. Subsequently, the same two authors independently reviewed the full texts of studies identified as possibly relevant. Disagreements at each stage were adjudicated by discuss with a third review author (VM).
Data extraction and management
Two review authors (ZL and LAP) independently extracted data from included studies into standardised spreadsheets. We recorded the following where reported: study design; population baseline characteristics, clinical dermatological characteristics such as psoriatic arthritis prevalence, Psoriasis Area and Severity Index (PASI), disease duration; comorbidities; mean PLT, MPV, PDW, PCT, IPF, and PMI measurements and standard deviation stratified into psoriasis vs control groups; and correlation of the aforementioned platelet parameters with PASI score. Where studies contrasted average platelet measurements between psoriasis and control groups, we standardised reported data as mean and standard deviation [23].
Assessment of methodological quality
Two review authors (ZL and LAP) independently assessed risk of bias of included studies using the Newcastle–Ottawa Quality Assessment Scale (NOS) for case–control studies [24], with discrepancies adjudicated by discussion with a third author (VM). The NOS consists of a linear ‘star’ scale ranging from 0 stars (worst) to 9 stars (best). Stars may be awarded in 8 different areas classified under 3 subscales of selection, comparability, and exposure. We graded a study with a star count of at least 7 as high quality, at least 5 as fair quality, and otherwise as poor quality [25,26,27].
Statistical analysis and data synthesis
We tabulated mean differences (MD) with associated confidence intervals (CI) and correlation coefficients for each included study grouped by platelet parameter, generated summary estimates with random-effects inverse-variance modelling, and graphically depicted findings using forest plots. We performed meta-analyses where studies were study numbers were sufficient and studies were not excessively clinically heterogeneous; otherwise, we performed qualitative descriptive analyses.
We used the I2 statistic to approximate statistical heterogeneity for each meta-analysis, and where possible used meta-regression to investigate potential sources of heterogeneity where this was significant (clinical judgement, I2 statistic > 50%, and where there were approximately ten or greater studies in the analysis) through inputting pre-specified covariates into a mixed-effects model [28]. We pre-specified the following covariates for meta-regression: study characteristics including retrospective vs prospective study design, methodological quality out of nine stars as per the Newcastle–Ottawa scale, sample size, and percentage male; patient attributes including average age and body mass index; clinical dermatological features such as PASI score, duration of disease, prevalence of psoriatic arthritis within psoriasis patients; as well as patient comorbidities such as prevalence of smoking, alcohol consumption, hypertension, diabetes mellitus, cardiovascular disease, metabolic syndrome, and dyslipidaemia in both psoriasis and control populations.
Where studies were assessed as overall poor quality on the NOS, and where such studies were included in quantitative meta-analysis, we performed sensitivity analyses to assess the influence of these studies by removing them from the meta-analysis. We also performed ‘leave-one-out’ sensitivity analyses for each meta-analysis to evaluate the impact of each single included study on pooled results.
Where there were at least 10 included studies in a meta-analysis, we generated contour enhanced funnel plots to formally assess for reporting bias [29]. We used Egger’s regression test to analyse potential funnel plot asymmetry, followed by visual inspection to identify the statistical significance of regions of potentially missing studies, to evaluate the likelihood of asymmetry being due to reporting bias as opposed to other factors such as poor methodological quality leading to spuriously inflated effects in smaller studies, true heterogeneity, artefact, or chance [30, 31].
We used the R [32] statistical package ‘metafor’ [33], Review Manager (RevMan) 5.4 [34], and Stata [35] to perform all analyses and generate figures.
Results
Search results
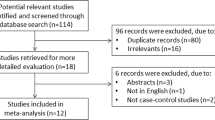
Our database searches returned 1,390 results, with 37 potentially relevant studies identified from other sources. After automatic deduplication, we screened the titles and abstracts of 1,079 unique studies. Fifty-nine studies underwent full-text review, from which 33 studies were included in the final review (Fig. 1).
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart. 25 full-text articles were excluded due to lack of platelet count, mean platelet volume, platelet distribution width, or plateletcrit reporting. 1 full-text article was excluded due to identical cohort analysed to included study
Description of included studies
Thirty-three studies [8, 9, 36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66] encompassing 6724 patients published between 2008 and 2021were included. All studies were case-control studies performed at a single centre. Detailed characteristics of included studies are explored in Table 1.
Methodological quality
Methodological quality was variable across studies, as assessed by the NOS. Thirteen studies [9, 36, 39,40,41, 43, 44, 51, 54, 56, 62, 64, 65] were regarded as being high quality, eighteen studies [8, 37, 38, 45, 46, 48,49,50, 52, 53, 55, 57,58,59,60,61, 63, 66] fair quality, and two studies [42, 47] were identified as poor quality. All studies attained 3 out of 3 stars in the exposure subscale; overall methodological quality was differentiated in the subscales of selection and comparability. Studies ranged from 1 to 4 stars in selection, with lower star ratings arising from concerns of case definition, representativeness of cases, and selection of controls. Studies ranged from 0 to 2 stars in comparability of cases and controls on the basis of design or analysis. The complete NOS assessment can be found in Online Resource 2.
Meta-analyses of platelet parameters
Platelet count.
Presence of psoriasis
From 24 studies [8, 37, 40,41,42, 44,45,46, 50,51,52, 54,55,56,57,58,59,60,61,62,63,64,65,66] involving 5,944 patients, we found that patients with psoriasis had a statistically significant increased PLT compared to controls (MD 12.86 × 109/L, 95% CI 6.34–19.39, p < 0.001) (Fig. 2). We performed a sensitivity analysis to remove one study [42] identified as being of poor methodological quality; resulting PLT differences were similar (MD 12.96 × 109/L, 95% CI 6.27–19.66, p < 0.001). Leave-one-out sensitivity analyses were not significant (Online Resource 3).
Statistical heterogeneity was substantial (I2 statistic 66%). We used meta-regression of pre-specified covariates to investigate this further, and found that prevalence of smoking, comorbid cardiovascular disease, hypertension, and dyslipidaemia were statistically significant positive modifiers of the MD (regression coefficients 62.97, 733.86, 61.86, 165.52; p-values 0.015, 0.020, 0.022 and 0.040 respectively). In particular, we found that smoking prevalence accounted for up to 100% of observed statistical heterogeneity (Online Resource 4).
Egger’s regression test for funnel plot asymmetry using a weighted regression with multiplicative dispersion model did not reveal significant asymmetry (p = 0.654); this was consistent with visual inspection (Online Resource 5).
Severity of psoriasis
From 9 studies [46, 50, 51, 54, 55, 57, 59, 62, 63] involving 2,731 patients, we found a weak correlation between PLT and PASI in patients with psoriasis (correlation coefficient 0.17, 95% CI 0.06–0.28, p = 0.003) (Fig. 3). There was a substantial degree of statistical heterogeneity (I2 statistic 69%); however, meta-regression was not possible due to insufficient study number for analysis. Similarly, formal testing for reporting bias was not possible due to low study numbers. Leave-one-out sensitivity analyses were not significant (Online Resource 6).
Mean platelet volume
Presence of psoriasis
From 26 studies [8, 9, 36, 38, 39, 41,42,43,44,45,46,47,48,49,50, 52,53,54,55,56,57,58,59, 61, 63, 66] involving 4,300 patients, we found that patients with psoriasis had a statistically significant increased MPV compared to controls (MD 0.61 fL, 95% CI 0.31–0.92, p < 0.001) (Fig. 4). We performed a sensitivity analysis to remove two studies [42, 47] identified as being of poor methodological quality; resulting MPV differences were similar (MD 0.69 fL, 95% CI 0.37–1.00, p < 0.001). Leave-one-out sensitivity analyses were not significant (Online Resource 7).
Statistical heterogeneity was considerable (I2 statistic 94%). We used meta-regression of pre-specified covariates to investigate this further, and found that percentage male and duration of psoriasis were statistically significant modifiers of the MD (regression coefficients 5.33, − 0.14; p-values < 0.001, 0.008 respectively). These accounted for up to 60% and 29% of observed statistical heterogeneity respectively in univariable analysis; however, residual heterogeneity remained considerable (Online Resource 8).
Egger’s regression test for funnel plot asymmetry using a weighted regression with multiplicative dispersion model did not reveal significant asymmetry (p = 0.889); this was consistent with visual inspection (Online Resource 9).
Severity of psoriasis
From 19 studies [8, 9, 39, 43, 45,46,47,48,49,50, 52,53,54,55, 57,58,59, 61, 63] involving 2,583 patients, we found a weak correlation between MPV and PASI in patients with psoriasis (correlation coefficient 0.36, 95% CI 0.22–0.49, p < 0.001) (Fig. 5). We performed a sensitivity analysis to remove one study [47] identified as being of poor methodological quality; resulting correlation was similar (correlation coefficient 0.37 fL, 95% CI 0.23–0.51, p < 0.001). Leave-one-out sensitivity analyses were not significant (Online Resource 10).
There was a considerable degree of statistical heterogeneity (I2 statistic 92%). We used meta-regression of pre-specified covariates to investigate this further, and found that percentage male was a statistically significant modifier of the correlation coefficient (regression coefficient 1.12; p-value 0.004). This accounted for up to 33% of observed statistical heterogeneity in univariable analysis; however, residual heterogeneity remained considerable (Online Resource 11).
Egger’s regression test for funnel plot asymmetry using a weighted regression with multiplicative dispersion model suggested significant asymmetry (p = 0.003). Visual inspection suggested potential missing studies in the bottom right hand side of the funnel plot (Online Resource 12). Given that most of this area contains regions of high significance as represented by the shaded triangles, the underlying cause of asymmetry is more likely true heterogeneity, artefact, or chance than reporting bias, though the latter cannot be excluded.
Platelet distribution width
Presence of psoriasis
From 8 studies [39, 46, 50, 52, 58, 59, 64, 66] involving 2,130 patients, we found no significant difference in PDW in patients with psoriasis compared to controls (MD 0.16%, 95% CI − 0.46–0.79, p = 0.610) (Fig. 6). There was a substantial degree of statistical heterogeneity (I2 statistic 95%); however, meta-regression was not possible due to insufficient study number for analysis. Similarly, formal testing for reporting bias was not possible due to low study numbers. Leave-one-out sensitivity analyses were not significant (Online Resource 13).
Severity of psoriasis
From 6 studies [39, 46, 50, 52, 58, 59] involving 802 patients, we found a weak correlation between PDW and PASI in patients with psoriasis (correlation coefficient 0.17, 95% CI 0.08–0.26, p < 0.001) (Fig. 7). Statistical heterogeneity was absent (I2 statistic 0%). Formal testing for reporting bias was not possible due to low study numbers. Leave-one-out sensitivity analyses were not significant (Online Resource 14).
Plateletcrit
Presence of psoriasis
From 3 studies [55, 58, 66] involving 1,348 patients, we found that patients with psoriasis had a statistically significant increased PCT compared to controls (MD 0.05%, 95% CI 0.01–0.09, p = 0.010) (Fig. 8). There was a substantial degree of statistical heterogeneity (I2 statistic 85%); however, meta-regression was not possible due to insufficient study number for analysis. Similarly, formal testing for reporting bias was not possible due to low study numbers.
Severity of psoriasis
One study [55] involving 300 patients reported a weak correlation between PCT and PASI in patients with psoriasis (correlation coefficient 0.11, 95% CI 0.00–0.22, p = 0.001).
Immature platelet fraction
Presence of psoriasis
One study [44] involving 63 patients reported no significant difference in IPF in patients with psoriasis compared to controls (MD −0.20%, 95% CI − 1.89–1.49, p = 0.890).
Severity of psoriasis
No studies reported on the correlation of IPF and PASI in patients with psoriasis.
Platelet mass index
Presence of psoriasis
One study [61] involving 520 patients reported a significant elevation in PMI in patients with psoriasis compared to controls (MD 295, 95% CI 190–400, p < 0.001).
Severity of psoriasis
One study [61] involving 520 patients did not find a significant correlation of PMI and PASI in patients with psoriasis (correlation coefficient − 0.02, 95% CI -0.13–0.09, p = 0.69).
Discussion
This systematic review and meta-analysis found that patients with psoriasis have elevated PLT, MPV, and PCT, but not PDW, measurements compared to controls. Moreover, PLT, MPV, and PDW were weakly correlated with PASI in patients with psoriasis. Further studies are needed to clarify the relationship between IPF and PMI and psoriasis, as well as the correlation between PDW, IPF, and PMI with PASI.
Included studies displayed variable methodological quality as assessed by NOS; however, sensitivity analyses removing two studies deemed to be poor quality and at high risk of bias showed robustness of our findings, and meta-regression of NOS methodological quality out of nine stars did not significantly modify effect size where it could be performed. Heterogeneity of included studies was considerable in many meta-analyses. Our meta-regression revealed cardiovascular disease and its risk factors of smoking, diabetes, and dyslipidaemia to be significant modifiers of the PLT mean difference in those with and without psoriasis, percentage male and duration of psoriasis to be significant modifiers of the MPV mean difference in those with and without psoriasis, and percentage male to be a significant modifier of the correlation coefficient between MPV and PASI. Future high-quality studies controlling for the aforementioned factors are needed to clarify the independence of our results against these potential confounding influences. Egger’s regression test for funnel plot asymmetry revealed statistical asymmetry in the analysis of correlation between MPV and PASI; however, visual inspection of the contour enhanced funnel plot showed potentially missing studies, if any, isolated to high significance regions on the plot. This suggested an increased likelihood of clinical and statistical heterogeneity between included studies as the underlying cause of funnel plot asymmetry, rather than reporting bias.
Our study extends the findings of a recent meta-analysis by Li et al. (2021) of 22 studies including 3287 patients which found that higher MPV and PDW, but not PLT, were associated with presence of psoriasis [67]. The discrepancies between PLT and PDW conclusions between studies is likely attributable to differences in number of included studies; indeed, our meta-analyses were able to include all 22 studies reported by Li et al. as well as an additional 11 relevant studies to investigate platelet indices in both presence and severity of psoriasis. Moreover, our study was able to review the state of evidence for other platelet parameters including PCT, IPF, and PMI, and found that PCT was elevated in patients with psoriasis.
Beyond their primary haemostatic role, platelets have been found to contribute to diverse immune regulatory processes. These include the expression of surface immune-related receptors such as P-selectin and CD40 ligand which facilitate leukocyte recruitment to sites of cutaneous endothelial inflammation, as well as the release of chemokines, pro-inflammatory factors, and cytokine-like molecules into the circulation upon activation [68, 69]. Studies suggest that platelet activation occurs in patients with inflammatory cutaneous disease such as psoriasis, urticaria, atopic dermatitis [70]. Berrettini et al. found that patients with psoriasis had a significantly higher plasma level of platelet chemokine β-thromboglobulin and incidence of spontaneous platelet hyperaggregability compared with control subjects [71]. Tamagawa-Mineoka et al. reported increased plasma levels of β-thromboglobulin and platelet factor 4 (another platelet chemokine) in patients with psoriasis which were both correlated with PASI and significantly reduced following successful treatment, suggesting a pathomechanistic contribution to disease activity [72]. It makes sense then that platelet function indices may be associated with psoriasis. MPV measures platelet size which increases with platelet activation [73]; as an acute phase reactant like C-reactive protein which can similarly be elevated in psoriasis [74], increases in PLT may be reflective of the underlying psoriatic inflammatory milieu; as a measure of the mass of platelets and proportional to both PLT and MPV, an increased PCT follows.
As a measure of variability in platelet size and shape, mounting evidence suggests that PDW may be a more specific marker of platelet activation than MPV, due to the former’s elevation in platelet anisocytosis after pseudopodia formation in platelet activation, but not in platelet distension following simple platelet swelling [19, 75]. While our study was able to demonstrate a relationship for MPV but not PDW, the interpretation of this finding is confounded by the disparate evidence base for the two biomarkers (26 vs 8 studies, respectively) and considerable between-study heterogeneity. Future studies are needed to validate the role of PDW in psoriasis.
Our results should be considered with the following limitations. Although we were able to perform meta-regressions to explore the effects of 13 pre-specified covariates on effect measures, and were able to identify significant effect modifiers, residual heterogeneity remained in all but the PLT meta-analysis. This persisting heterogeneity may be attributable to systemic differences in unreported study population or clinical factors. Additionally, low study numbers in some analyses meant that even though meta-analysis could be performed, both meta-regression assessment of heterogeneity and funnel plot assessment of reporting bias could not be performed, despite considerable heterogeneity. All included studies were single centre; large, high quality multicentre studies could improve external validity. Finally, the inconsistent reporting of treatment regimens used by patients with psoriasis meant that we were unable to account for the influence of specific immunosuppressive regimens (e.g. topical therapy vs phototherapy vs oral immunosuppressives vs biologics) on platelet indices.
This study identifies multiple opportunities for future research. Cheap and accessible haematological biomarkers of psoriasis presence and severity are potentially useful adjuncts to the purely clinical scoring systems in use currently—the PASI and the Psoriasis Global Assessment (PGA) scores—and indeed may be incorporated into such systems to further refine their predictive potential [76]. Whether or not platelet indices could contribute to disease diagnosis in clinically or pathologically dubious cases, or be prognostic for future adverse outcomes such as the development of psoriatic arthritis, cardiovascular disease, major adverse cardiac events, or mortality remains poorly understood. For platelet indices to be easily implementable in the clinical context, cut-off values distinguishing between normality and elevation should be developed and validated. Lastly, while the scope of this review was limited to specific platelet indices, other full blood examination biomarkers such as the neutrophil–lymphocyte ratio, platelet-lymphocyte ratio, and red cell distribution width have been found to be disturbed in psoriasis and systemic conditions associated with inflammation [10, 77,78,79,80,81]. The incorporation of clinical, pathological, and haematological biomarkers into validated predictive models could enhance all aspects of disease management and patient care.
This systematic review and meta-analysis found that PLT, MPV, and PCT are significantly elevated in patients with psoriasis compared to controls, and that PLT, MPV, and PDW are weakly correlated with PASI. Future studies are needed to evaluate the independent diagnostic and prognostic potentials of these biomarkers in patients with psoriasis.
References
Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205–12.
Farley E, Menter A. Psoriasis: comorbidities and associations. G Ital Dermatol Venereol. 2011;146:9–15.
Coumbe AG, Pritzker MR, Duprez DA. Cardiovascular risk and psoriasis: beyond the traditional risk factors. Am J Med. 2014;127:12–8.
Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–35.
Sharma G, Berger JS. Platelet activity and cardiovascular risk in apparently healthy individuals: a review of the data. J Thromb Thrombolysis. 2011;32:201–8.
Trip MD, Cats VM, van Capelle FJ, Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med. 1990;322:1549–54.
Willoughby S, Holmes A, Loscalzo J. Platelets and cardiovascular disease. Eur J Cardiovasc Nurs. 2002;1:273–88.
Canpolat F, Akpinar H, Eskioglu F. Mean platelet volume in psoriasis and psoriatic arthritis. Clin Rheumatol. 2010;29:325–8.
Saleh HM, Attia EA, Onsy AM, Saad AA, Abd Ellah MM. Platelet activation: a link between psoriasis per se and subclinical atherosclerosis–a case-control study. Br J Dermatol. 2013;169:68–75.
Paliogiannis P, Satta R, Deligia G, et al. Associations between the neutrophil-to-lymphocyte and the platelet-to-lymphocyte ratios and the presence and severity of psoriasis: a systematic review and meta-analysis. Clin Exp Med. 2019;19:37–45.
Liu Z, Perry LA, Penny-Dimri JC, et al. Donor cardiac troponin for prognosis of adverse outcomes in cardiac transplantation recipients: a systematic review and meta-analysis. Transplant Direct. 2022;8:e1261.
Borg Caruana C, Jackson SM, Ngyuen Khuong J, et al. Systematic review and meta-analysis of postoperative troponin as a predictor of mortality and major adverse cardiac events after vascular surgery. J Vasc Surg. 2020;72:1132-43 e1.
Perry LA, Liu Z, Loth J, et al. Perioperative neutrophil-lymphocyte ratio predicts mortality after cardiac surgery: systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2022;36:1296–303.
Jackson SM, Perry LA, Borg C, et al. Prognostic significance of preoperative neutrophil-lymphocyte ratio in vascular surgery: systematic review and meta-analysis. Vasc Endovasc Surg. 2020;54:697–706.
Liu Z, Perry LA, Penny-Dimri JC, et al. Prognostic significance of elevated troponin in adult heart transplant recipients: a systematic review and meta-analysis. Exp Clin Transplant. 2022.
Chu SG, Becker RC, Berger PB, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–56.
Chandrashekar L, Rajappa M, Sundar I, et al. Platelet activation in chronic urticaria and its correlation with disease severity. Platelets. 2014;25:162–5.
Tamer F, Yuksel ME, Avci E. Is mean platelet volume an inflammatory marker in acne patients treated with isotretinoin? Acta Dermatovenerol Alp Pannonica Adriat. 2019;28:65–9.
Liu Z, Perry LA, Edwards TL. Association between platelet indices and retinal vein occlusion: a systematic review and meta-analysis. Retina. 2021;41:238–48.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
Covidence systematic review software. Veritas Health Innovation, Melbourne, Australia. www.covidence.org.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Buckthorpe M, Wright S, Bruce-Low S, et al. Recommendations for hamstring injury prevention in elite football: translating research into practice. Br J Sports Med. 2019;53:449–56.
Islam MM, Iqbal U, Walther B, et al. Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology. 2016;47:181–91.
Ji S, Zhang J, Fan X, et al. The relationship between mean platelet volume and diabetic retinopathy: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11:25.
Chapter 10: Analysing data and undertaking meta-analyses. In: Deeks JJ, Higgins JPT, Altman DG, eds. Cochrane Handbook for Systematic Reviews of Interventions. 6.1 edn. Cochrane, 2020.
Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Page MJ, Higgins JPT, Sterne JAC, eds. Cochrane Handbook for Systematic Reviews of Interventions. 6.1 edn. Cochrane, 2020.
Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55.
Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
Review Manager (RevMan). 5.4 edn. The Cochrane Collaboration, 2020.
Stata Statistical Software. Release 17. College Station, TX: StataCorp LLC; 2021.
Ahmad Z, Akhtar SJ, Maan MA, Khalid U, Hussain A. Comparison of mean platelet volume in patients with psoriasis and healthy individuals. J Pak Assoc Dermatol. 2014;24:4–7.
Ataseven A, Bilgin AU, Kurtipek GS. The importance of neutrophil lymphocyte ratio in patients with psoriasis. Mater Sociomed. 2014;26:231–3.
Çerman AA, Karabay EA, Altunay IK. Psoriazisli Hastalarda Nötrofil Lenfosit Oranı ve Ortalama Trombosit Hacminin Değerlendirilmesi/Evaluation of neutrophil-lymphocyte ratio and mean platelet volume in patients with psoriasis. Şişli Etfal Hastanesi Tip Bülteni. 2016;50:137–41.
Chandrashekar L, Rajappa M, Revathy G, et al. Is enhanced platelet activation the missing link leading to increased cardiovascular risk in psoriasis? Clin Chim Acta. 2015;446:181–5.
Dincer Rota D, Tanacan E. The utility of systemic-immune inflammation index for predicting the disease activation in patients with psoriasis. Int J Clin Pract. 2021;75:e14101.
Dogan S, Atakan N. Red blood cell distribution width is a reliable marker of inflammation in plaque psoriasis. Acta Dermatovenerol Croat. 2017;25:26–31.
Erek Toprak A, Ozlu E, Uzuncakmak TK, Yalcinkaya E, Sogut S, Karadag AS. Neutrophil/lymphocyte ratio, serum endocan, and nesfatin-1 levels in patients with psoriasis vulgaris undergoing phototherapy treatment. Med Sci Monit. 2016;22:1232–7.
Farag AGA, Zytoon AA, Habib MS, et al. Mean platelet volume: an immanent predictor of subclinical atherosclerosis in psoriatic patients compared with interleukin-1α and interleukin-6. J Egypt Women Dermatol Soc. 2018;15:80–7.
Garshick MS, Tawil M, Barrett TJ, et al. Activated platelets induce endothelial cell inflammatory response in psoriasis via COX-1. Arterioscler Thromb Vasc Biol. 2020;40:1340–51.
Hammad R, Hamdino M, El-Nasser AM. Role of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, mean platelet volume in egyptian patients with psoriasis vulgaris. Egypt J Immunol. 2020;27:157–68.
Hancer HS, Alatas ET. Evaluation of mean platelet volume, neutrophil/lymphocyte ratio and platelet/lymphocyte ratio relationship with disease severity and metabolic syndrome in patients with psoriasis vulgaris. Ann Med Res. 2020;27:2503–9.
Isik S, Kilic S, Ogretmen Z, et al. The correlation between the psoriasis area severity index and ischemia-modified albumin, mean platelet volume levels in patients with psoriasis. Postepy Dermatol Alergol. 2016;33:290–3.
Karabudak O, Ulusoy RE, Erikci AA, Solmazgul E, Dogan B, Harmanyeri Y. Inflammation and hypercoagulable state in adult psoriatic men. Acta Derm Venereol. 2008;88:337–40.
Kilic S, Resorlu H, Isik S, et al. Association between mean platelet volume and disease severity in patients with psoriasis and psoriatic arthritis. Postepy Dermatol Alergol. 2017;34:126–30.
Kim DS, Lee J, Kim SH, Kim SM, Lee MG. Mean platelet volume is elevated in patients with psoriasis vulgaris. Yonsei Med J. 2015;56:712–8.
Kim DS, Shin D, Lee MS, et al. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol. 2016;43:305–10.
Korkmaz S. Mean platelet volume and platelet distribution width levels in patients with mild psoriasis vulgaris with metabolic syndrome. Postepy Dermatol Alergol. 2018;35:367–71.
Mahrous E. The relationship between platelet volume and risk of atherosclerosis in patients with psoriasis. Egypt J Dermatol Venerol. 2018;38:29–36.
Ozkur E, Seremet S, Afsar FS, Altunay IK, Calikoglu EE. Platelet count and mean platelet volume in psoriasis patients. Sisli Etfal Hastan Tip Bul. 2020;54:58–61.
Pektas SD, Alatas ET, Yilmaz N. Plateletcrit is potential biomarker for presence and severity of psoriasis vulgaris. Acta Medica Mediterranea. 2016;32:1785–90.
Polat M, Bugdayci G, Kaya H, Oguzman H. Evaluation of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in Turkish patients with chronic plaque psoriasis. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:97–100.
Raghavan V, Radha RKN, Rao RK, Kuberan A. A correlative study between platelet count, mean platelet volume and red cell distribution width with the disease severity index in psoriasis patients. J Clin Diagn Res. 2017;11:13–6.
Sharma S, Yadav S, Naeem R, Sarin N, Khurana V, Singh S. Platelet count and indices in patients with psoriasis: are they associated with disease severity? Arch Med Health Sci. 2020;8:221–4.
Sirin MC, Korkmaz S, Erturan I, et al. Evaluation of monocyte to HDL cholesterol ratio and other inflammatory markers in patients with psoriasis. An Bras Dermatol. 2020;95:575–82.
Tamagawa-Mineoka R, Katoh N, Kishimoto S. Platelet activation in patients with psoriasis: increased plasma levels of platelet-derived microparticles and soluble P-selectin. J Am Acad Dermatol. 2010;62:621–6.
Ünal M. Platelet mass index is increased in psoriasis. A possible link between psoriasis and atherosclerosis. Arch Med Sci Atheroscler Dis. 2016;1:e145–9.
Wang WM, Wu C, Gao YM, Li F, Yu XL, Jin HZ. Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and other hematological parameters in psoriasis patients. BMC Immunol. 2021;22:64.
Yavuz GÖ, Yavuz İH. Novel inflammatory markers in patients with psoriasis. East J Med. 2019;24:63–8.
Yorulmaz A, Hayran Y, Akpinar U, Yalcin B. Systemic immune-inflammation index (SII) predicts increased severity in psoriasis and psoriatic arthritis. Curr Health Sci J. 2020;46:352–7.
Yurtdas M, Yaylali YT, Kaya Y, Ozdemir M, Ozkan I, Aladag N. Neutrophil-to-lymphocyte ratio may predict subclinical atherosclerosis in patients with psoriasis. Echocardiography. 2014;31:1095–104.
Zhou J, Li Y, Guo X. Predicting psoriasis using routine laboratory tests with random forest. PLoS ONE. 2021;16:e0258768.
Li L, Yu J, Zhou Z. Platelet-associated parameters in patients with psoriasis: A PRISMA-compliant systematic review and meta-analysis. Med (Baltimore). 2021;100:e28234.
Smyth SS, McEver RP, Weyrich AS, et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759–66.
Katoh N. Platelets as versatile regulators of cutaneous inflammation. J Dermatol Sci. 2009;53:89–95.
Kasperska-Zajac A, Brzoza Z, Rogala B. Platelet function in cutaneous diseases. Platelets. 2008;19:317–21.
Berrettini M, Parise P, Constantini V, Grasselli S, Nenci GG. Platelet activation in psoriasis. Thromb Haemost. 1985;53:195–7.
Tamagawa-Mineoka R, Katoh N, Ueda E, Masuda K, Kishimoto S. Elevated platelet activation in patients with atopic dermatitis and psoriasis: increased plasma levels of beta-thromboglobulin and platelet factor 4. Allergol Int. 2008;57:391–6.
Park Y, Schoene N, Harris W. Mean platelet volume as an indicator of platelet activation: methodological issues. Platelets. 2002;13:301–6.
Beygi S, Lajevardi V, Abedini R. C-reactive protein in psoriasis: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:700–11.
Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14:28–32.
Wells G, Becker JC, Teng J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68:954–60.
Liu Z, Nguyen Khuong J, Borg Caruana C, et al. The prognostic value of elevated perioperative neutrophil-lymphocyte ratio in predicting postoperative atrial fibrillation after cardiac surgery: a systematic review and meta-analysis. Heart Lung Circ. 2020;29:1015–24.
Gisondi P, Geat D, Lippi G, Montagnana M, Girolomoni G. Increased red blood cell distribution width in patients with plaque psoriasis. J Med Biochem. 2021;40:199–201.
Liu Z, Perry LA, Penny-Dimri JC, et al. The association of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio with retinal vein occlusion: a systematic review and meta-analysis. Acta Ophthalmol. 2021.
Campbell R, Khuong JN, Liu Z, et al. Perioperative gabapentinoid use lowers short-term opioid consumption following lower limb arthroplasty: systematic review and meta-analysis. J Opioid Manag. 2021;17:251–72.
Ramson DM, Gao H, Penny-Dimri JC, et al. Duration of post-operative antibiotic treatment in acute complicated appendicitis: systematic review and meta-analysis. ANZ J Surg. 2021;91:1397–404.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Zhengyang Liu and Luke A Perry contributed to the study conception and design. Material preparation and data collection were performed by Zhengyang Liu; all authors contributed to data-analysis and interpretation. The first draft of the manuscript was written by Zhengyang Liu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This is a systematic review and meta-analysis investigating published study-level data; hence, no ethical approval is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Z., Perry, L.A. & Morgan, V. The association between platelet indices and presence and severity of psoriasis: a systematic review and meta-analysis. Clin Exp Med 23, 333–346 (2023). https://doi.org/10.1007/s10238-022-00820-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00820-5