Abstract
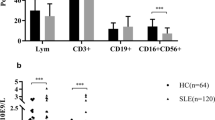
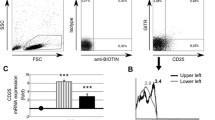
Expressed on the cell surface of most of NK cells and some T cells, CD161 has been shown to deliver inhibitory signal in human NK cells. To determine whether the CD161-expressing cell quantities and the cell surface expression levels of CD161 in NK and T cells were altered in systemic lupus erythematosus (SLE) patients, we analyzed the CD3, CD56 and CD161 expression patterns of peripheral blood lymphocytes by flow cytometric analysis to identify different NK and T cell subpopulations. The cell surface expression levels of CD161 were estimated by the mean florescence intensities (MFIs) of CD161. It was found that SLE patients had lower frequencies of CD161+CD56+CD3− and CD161+CD56+CD3+ cells among the lymphocyte population than normal controls, whereas the frequencies of CD161−CD56+CD3− and CD161+CD56−CD3+ cells were not statistically different between two groups. In addition, SLE patients also had decreased absolute counts of all CD161-expressing NK cells and T cells and had reduced frequencies of CD161+ cells in CD56+CD3−, CD56+CD3+ and CD56−CD3+ cell populations. Moreover, SLE patients had reduced MFIs of CD161 in CD161+CD56+CD3+ and CD161+CD56−CD3+, but not CD161+CD56+CD3−, cell populations. Our results indicated that CD161-expressing cell frequency and the CD161 expression levels were reduced in some NK and T cell subpopulations of SLE patients, suggesting possible important role of CD161 and CD161-expressing immune cells in the SLE pathogenesis.






Similar content being viewed by others
References
Sibbitt WL Jr, Mathews PM, Bankhurst AD. Natural killer cell in systemic lupus erythematosus. Defects in effector lytic activity and response to interferon and interferon inducers. J Clin Invest. 1983;71:1230–9.
Yabuhara A, Yang FC, Nakazawa T, et al. A killing defect of natural killer cells as an underlying immunologic abnormality in childhood systemic lupus erythematosus. J Rheumatol. 1996;23:171–7.
Stohl W, Elliott JE, Hamilton AS, Deapen DM, Mack TM, Horwitz DA. Impaired recovery and cytolytic function of CD56+ T and non-T cells in systemic lupus erythematosus following in vitro polyclonal T cell stimulation. Studies in unselected patients and monozygotic disease-discordant twins. Arthritis Rheum. 1996;39:1840–51.
Erkeller-Yuksel FM, Lydyard PM, Isenberg DA. Lack of NK cells in lupus patients with renal involvement. Lupus. 1997;6:708–12.
Riccieri V, Spadaro A, Parisi G, et al. Down-regulation of natural killer cells and of γ/δ T cells in systemic lupus erythematosus. Does it correlate to autoimmunity and to laboratory indices of disease activity? Lupus. 2000;9:333–7.
Park Y-W, Kee S-J, Cho Y-N, et al. Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1753–63.
Cho Y-N, Kee S-J, Lee S-J, et al. Numerical and functional deficiencies of natural killer T cells in systemic lupus erythematosus: their deficiency related to disease activity. Rheumatology. 2011;50:1054–63.
Hervier B, Beziat V, Haroche J, et al. Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon-γ production in patients with active disease. Arthritis Rheum. 2011;63:1698–706.
Puxeddu I, Bongiorni F, Chimenti D, et al. Cell surface expression of activating receptors and co-receptors on peripheral blood NK cells in systemic autoimmune diseases. Scand J Rheumatol. 2012;41:298–304.
Henriques A, Teixeira L, Inês L, et al. NK cells dysfunction in systemic lupus erythematosus: relation to disease activity. Clin Rheumatol. 2013;32:805–13.
Shi FD, Van Kaer L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nat Rev Immunol. 2006;6:751–60.
Wu W, Shi S, Ljunggren H-G, et al. NK cells inhibit T-bet-deficient, autoreactive Th17 cells. Scand J Immunol. 2012;76:559–66.
Fogel LA, Yokoyama WM, French AR. Natural killer cells in human autoimmune disorders. Arthritis Res Ther. 2013;15:216.
Mesci A, Ljutic B, Makrigiannis AP, Carlyle JR. NKR-P1 biology: from prototype to missing self. Immunol Res. 2006;35:13–26.
Lanier LL, Chang C, Phillips JH. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol. 1994;153:2417–28.
Takahashi T, Dejbakhsh-Jones S, Strober S. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. J Immunol. 2006;176:211–6.
Billerbeck E, Kang Y-H, Walker L, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci USA. 2010;107:3006–11.
Cosmi L, De Palma R, Santarlasci V, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–16.
Maggi L, Santarlasci V, Capone M, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol. 2010;40:2174–81.
Pesenacker AM, Bending D, Ursu S, Wu Q, Nistala K, Wedderburn LR. CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood. 2013;121:2647–58.
Aldemir H, Prod’homme V, Dumaurier M-J, et al. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol. 2005;175:7791–5.
Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol. 2005;175:7796–9.
Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7.
Bombardier C, Gladman D, Urowitz M, Caron D, Chang C. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40.
Mitsuo A, Morimoto S, Nakiri Y, et al. Decreased CD161+CD8+ T cells in the peripheral blood of patients suffering from rheumatic diseases. Rheumatology. 2006;45:1477–84.
Chalan P, Kroesen B-J, van der Geest KSM, et al. Circulating CD4+CD161+ T lymphocytes are increased in seropositive arthralgia patients but decreased in patients with newly diagnosed rheumatoid arthritis. PLoS One. 2013;8:e79370.
Li W-X, Pan H-F, Hu J-L, et al. Assay of T- and NK-cell subsets and the expression of NKG2A and NKG2D in patients with new-onset systemic lupus erythematosus. Clin Rheumatol. 2010;29:315–23.
Bai Y, Zhang Y, Yang Q, et al. The aberrant expression of stimulatory and inhibitory killer immunoglobulin-like receptors in NK- and NKT-cells contributes to lupus. Clin Lab. 2014;60:717–27.
Schepis D, Gunnarsson I, Eloranta M-L, et al. Increased proportion of CD56bright natural killer cells in active and inactive systemic lupus erythematosus. Immunology. 2009;126:140–6.
Bedoya SK, Lam B, Lau K, Larkin J III. Th17 cells in immunity and autoimmunity. Clin Dev Immunol. 2013;2013:986789.
Acknowledgments
I thank Yanfeng Lu for review of the manuscript. This study was supported by a grant from the joined research found of Cathay General Hospital and National Taiwan University and by a grant from the National Science Council of Taiwan (Grant#101-2314-B-281-006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Lin, YL., Lin, SC. Analysis of the CD161-expressing cell quantities and CD161 expression levels in peripheral blood natural killer and T cells of systemic lupus erythematosus patients. Clin Exp Med 17, 101–109 (2017). https://doi.org/10.1007/s10238-015-0402-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-015-0402-1