Abstract
In order to determine the glycosylation pattern for IgD, and to examine whether there are changes in the pattern of IgD and IgA1 O-glycosylation in patients with hyperimmunoglobulinaemia D and periodic fever syndrome (HIDS) during acute febrile attacks and during periods of quiescence, serum was obtained from 20 patients with HIDS and 20 control subjects. In the HIDS group, serum was obtained either during an acute febrile episode (n = 9) or during a period of quiescence (n = 11). The O-glycosylation profiles of native and desialylated IgA1 and IgD were measured in an ELISA-type system using the lectins Helix aspersa and peanut agglutinin, which bind to alternative forms of O-glycan moieties. IgD is more heavily O-galactosylated and less O-sialylated than IgA1 in healthy subjects. HIDS is associated with more extensive O-galactosylation of IgD and a reduction in O-sialylation of both IgD and IgA1. These changes are present both during acute febrile attacks and periods of quiescence. The T cell IgD receptor is a lectin with binding affinity for the O-glycans of both IgD and IgA1. The observed changes in IgD and IgA1 O-glycosylation are likely to have a significant effect on IgD/IgA1–T cell IgD receptor interactions including basal immunoglobulin synthesis, and possibly myeloid IgD receptor-mediated cytokine release.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperimmunoglobulinaemia D and periodic fever syndrome (HIDS) is one of a group of hereditary periodic fever syndromes including familial Mediterranean fever (FMF); TNF-receptor-associated periodic syndrome (TRAPS); cryopyrin-associated periodic syndrome (CAPS) [1]. These autoinflammatory syndromes are characterised by recurrent episodes of fever accompanied by diverse systemic inflammatory symptoms which may include lymphadenopathy, abdominal discomfort, diarrhoea, vomiting, chest pain, joint involvement and skin lesions.
Hyperimmunoglobulinaemia D and periodic fever syndrome is inherited as an autosomal recessive disorder caused by partial deficiency of the enzyme mevalonate kinase (MVK), usually as a result of a compound heterozygotic missense mutation in the MVK gene on chromosome 12 [2]. MVK catalyses the ATP-dependent phosphorylation of mevalonate to produce 5-phosphomevalonate and is a key component in isoprenoid/cholesterol metabolism. Although the genetic defect has been well characterised, the mechanism by which a partial deficiency of MVK causes inflammatory attacks remains unclear.
The triggering of febrile attacks by vaccination, minor trauma, surgery and stress has led to the suggestion that regulation of the immune response may be abnormal in HIDS. There is evidence for defective lymphocyte apoptosis in HIDS supporting the notion that there are abnormalities of immune regulation and an inability to limit immunological responses in affected individuals [3].
Hyperimmunoglobulinaemia D and periodic fever syndrome is associated with high levels of circulating immunoglobulin, in particular IgD (>100 IU/ml or 14 mg/dl) and, in most patients IgA1 [4, 5]. The pathogenic significance of high IgD and IgA1 levels remains unclear, and while high immunoglobulin levels may simply reflect persistent dysregulated immune activation, we hypothesised that concurrent changes in immunoglobulin structure, in particular glycosylation, may play a role in potentiating the generalised inflammatory attacks characteristic of HIDS.
Glycosylation plays an important role in production, maintenance, handling and function of all glycoproteins and occurs during and immediately after protein synthesis [6]. There are two distinct forms of protein glycosylation, N-linked and O-linked. N-linked carbohydrates, linked to asparagine residues, are by far the most common, and usually consist of complex, branched chains [7]. Changes in immunoglobulin N-linked glycosylation are thought to contribute to the pathogenesis of a variety of autoimmune diseases including rheumatoid arthritis, SLE, Crohn’s disease, primary Sjögrens syndrome and psoriatic arthropathy [8].
O-linked sugars are more commonly associated with membrane-bound proteins and are less frequently found in serum proteins. IgA1 and IgD are unusual in that they both possess both N-linked and O-linked sugars. We and many others have described a number of changes in IgA1 O-glycosylation that are important in the pathogenesis of IgA nephropathy (IgAN) and Henoch Schönlein nephritis, two common patterns of glomerulonephritis [9]. Changes in O-linked galactosylation and sialylation have been shown to promote IgA1 self aggregation and formation of antigen–antibody complexes with IgG antibodies directed against IgA1 hinge epitopes. High circulating levels of polymeric IgA1 have also been described in HIDS although the mechanism for this is unknown [5]. Aberrantly, O-glycosylated IgA1 is also more likely to activate complement and IgA1 O-glycosylation also influences myeloid IgA receptor binding and IgA-induced cellular activation.
In order to determine whether O-linked glycosylation of IgA1 and IgD is abnormal in HIDS and whether changes in O-glycosylation might potentiate the generalised inflammatory attacks characteristic of HIDS, we measured the extent of hinge region O-galactosylation and O-sialylation of IgA1 and IgD in patients with HIDS both during acute febrile attacks and during periods of quiescence.
Materials and methods
Subjects
We studied 20 patients with HIDS (11 male, median age 29, range 18–61 years) and 20 healthy controls (13 male, median age 35, range 20–55 years). All patients showed the relevant pathogenic mutations (Table 1) and all participants gave informed consent for inclusion in the study. Venous blood was obtained either during an acute febrile episode (n = 9) or during a period of quiescence (n = 11) and serum separated and stored in aliquots at −80°C until required.
Materials
Unless otherwise stated, all the reagents and materials were purchased from Sigma Chemical Co., Poole, Dorset, UK. Polyclonal anti-IgA, anti-IgD and horseradish-peroxidase-conjugated anti-mouse antibodies, monoclonal anti-IgD and OPD substrate tablets were obtained from Dako Ltd (Ely, UK).
Measurement of serum IgA1 and IgD concentrations by ELISA
Serum levels of IgA1 and IgD were measured using specific ELISAs as published previously [10]. In brief, 96-well immunoplates were coated with rabbit anti-human antibodies to IgA1 and IgD. After washing with PBS containing an additional 0.4 M NaCl and 0.1% Tween 20, excess binding sites were blocked with 2% bovine serum albumin in PBS, plates were washed again and 50 μl aliquots of standard and test serum samples, diluted in PBS, were applied to duplicate wells. Standard curves were set up on each plate, using serial dilutions of a commercial human immunoglobulin calibrator preparation (The Binding Site, Birmingham, UK) ranging from 1 μg/ml to 1 ng/ml IgA1 or IgD. Serum samples were used at pre-optimised dilutions for each assay. After overnight incubation and further washing, secondary antibodies to human IgA1 (1:1,000) or IgD (1:250) were added for 2 h, plates were washed and horseradish peroxidase-conjugated anti-mouse immunoglobulin antibody applied to the plates. Plates were washed and developed with 1,2-phenylenediamine dihydrochloride (OPD)/H2O2 substrate solution and the results read on a densitometer at 492 nm (Titertek Multiscan, ICN Flow). Standard curves were constructed for each plate and sample values read from the standard curves, excluding samples which did not fall on the linear part of the curves; these were repeated at a more suitable dilution.
Lectin binding assays
In order to compare the O-glycosylation profiles of serum IgA1 and IgD in HIDS and healthy subjects, the binding of the lectins from Helix aspersa (HA) and peanut agglutinin (PNA) were measured using our previously published ELISA method [11]. HA recognises terminal O-linked N-acetylgalactosamine (GalNAc), and samples with lower terminal galactosylation and sialylation show higher HA binding. PNA binds to the core 1 disaccharide Gal-GalNAc, and is extremely inhibited by the presence of sialic acid. These assays correlate well with other more precise methods of immunoglobulin O-glycosylation analysis [12], and provide an excellent screening method for comparing the O-glycosylation patterns of multiple test samples.
Immunoplates were coated with anti-IgA1 or anti-IgD, blocked with BSA and diluted test serum samples applied to duplicate wells on a series of replicate immunoplates. We had previously shown that for IgA1, lectin binding was proportional to IgA1 over a concentration range of 0.1–100 μg/ml. In preliminary experiments for this study, this was also shown to be true for IgD lectin binding. Therefore, the test samples used in these assays were diluted to achieve IgA1 and IgD concentrations of approximately 1 μg/ml, and replicate plates developed with HA and PNA lectins, and also with anti-IgA1 or anti-IgD as appropriate, followed by anti-mouse-HRP as for ELISA. After overnight incubation and further washing, 50 μl/well-biotinylated HA and PNA lectins were applied to replicate plates at 1:250 and 1:500 in PBS, respectively, incubated for 90 min at room temperature, washed again, and lectin binding detected with 50 μl/well horseradish peroxidase-conjugated avidin. The results were expressed as mean absorbance at 492 nm of duplicate wells, with each lectin binding result adjusted for IgA1 or IgD binding absorbance to correct for minor variations in the immunoglobulin concentrations of the different samples.
Desialylation of IgA1 and IgD
The presence of terminal sialic acid moieties on the O-glycans of IgA1 and IgD can mask lectin binding to the inner chain and confuse interpretation of the results of these assays. Therefore, we compared lectin binding of native and desialylated IgA1 and IgD. Replicate immunoplates were coated with primary antibodies, blocked, and diluted serum samples applied as described above. After allowing capture of IgA1 or IgD for 24 h at 4°C, one set of plates was washed and desialylated with 50 μl/well Clostridium perfringens neuraminidase (New England Biolabs, Hitchin, UK) at 100 U/ml in 50 mM sodium citrate buffer, pH 6.0 for 18 h at 37°C. A parallel set of plates were left untreated at 4°C as native immunoglobulin. After this step, the lectin-binding assays were continued exactly as described above.
Statistical analysis
Serum IgA1 and IgD concentrations and lectin-binding results for the HIDS and control groups were expressed as mean ± standard error of the mean (SEM). Unpaired t-tests were used to compare the two subject groups, and paired t-tests to compare the lectin binding of native and desialylated IgA1 and IgD within the subject groups.
Results
Serum IgD and IgA1 levels
Hyperimmunoglobulinaemia D and periodic fever syndrome patients had extremely high serum IgD levels compared to controls (mean μg/ml IgD ± SEM: HIDS 299.9 ± 40.7, controls 3.94 ± 0.54, P < 0.001). Serum IgA1 was also modestly raised in the HIDS group (mean mg/ml IgA1 ± SEM: HIDS 3.05 ± 0.33, controls 1.56 ± 0.18, P < 0.001). Consistent with other reports IgD levels did not correlate with IgA1 levels and there was no significant difference in serum IgD and IgA1 levels during a febrile episode compared to periods of remission [4, 5].
O-glycosylation of serum IgD
Helix aspersa (recognises free terminal GalNAc residues) showed virtually no binding to native IgD from either healthy subjects or HIDS patients, with no difference between the groups (Fig. 1a). This indicates either that IgD carries no O-glycans, or that the O-linked GalNAc is completely masked by terminal galactose and/or sialic acid.
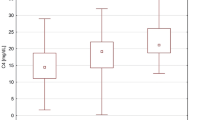
Lectin binding to the O-glycans of IgD and IgA1 in HIDS and healthy subjects. Binding of the lectins HA and PNA to native and desialylated IgD (a and b) and IgA1 (c and d) in patients with HIDS (open bars) and controls (black bars). Patients with HIDS were separated into those whose serum samples were collected during a febrile attack (HIDS-f) and those collected during a period of quiescence (HIDS-q). HIDS was associated with more extensive O-galactosylation of IgD and a reduction in O-sialylation of both IgD and IgA1, and these changes were present both during acute febrile attacks and periods of quiescence
Peanut agglutinin (recognises unsialylated Gal-GalNAc) bound to native IgD from both groups, demonstrating that IgD is O-glycosylated (Fig. 1b). The absence of HA binding therefore, indicates that the moieties are heavily galactosylated. Native IgD in HIDS exhibited higher PNA binding than controls (mean A492 PNA/A492 IgD ± SEM: HIDS 0.896 ± 0.086, healthy subjects 0.431 ± 0.043, P < 0.001), suggesting that IgD is less O-sialylated in HIDS.
Desialylation of IgD prior to lectin binding (Fig. 1a, b) resulted in a significantly lower fold increase in both HA and PNA lectin binding to IgD in HIDS than in healthy subjects (mean fold increase after desialylation ± SEM: HA HIDS 2.14 ± 0.096, control 3.07 ± 0.175, P < 0.001; PNA HIDS 2.27 ± 0.22, healthy subjects 4.83 ± 0.55, P < 0.001), confirming lower sialylation in HIDS.
Overall, these results indicate that in HIDS, serum IgD is more heavily O-galactosylated but less O-sialylated than in healthy subjects. These changes were present both during a febrile attack and convalescence.
O-glycosylation of serum IgA1
Helix aspersa lectin bound to native serum IgA1 in both HIDS and controls, demonstrating that this isotype is less completely O-galactosylated than IgD, with more GalNAc moieties exposed (Fig. 1c). HA binding to native IgA1 was higher in HIDS than controls (mean A492 HA/A492 IgA1 ± SEM: HIDS 0.953 ± 0.051, healthy subjects 0.742 ± 0.036, P = 0.0017).
Peanut agglutinin binding to native IgA1 (Fig. 1d) was also higher in HIDS than healthy subjects (mean A492 PNA/A492 IgA1 ± SEM: HIDS 0.515 ± 0.065, healthy subjects 0.202 ± 0.013, P < 0.001).
Desialylation of IgA1 increased both HA and PNA binding and removed the differences between HIDS and healthy subjects, indicating that the higher HA and PNA binding of native IgA1 in HIDS is due to differences in O-sialylation rather than O-galactosylation (Fig. 1c, d). This reduced O-sialylation is supported by the lower fold increases in lectin binding to IgA1 after desialylation in HIDS than in controls (mean fold increase after desialylation ± SEM: HA HIDS 1.58 ± 0.07, healthy subjects 1.92 ± 0.08, P = 0.028; PNA HIDS 4.62 ± 0.43, healthy subjects 9.86 ± 0.65, P < 0.001). Again, these changes were present both during a febrile attack and convalescence.
Discussion
There is increasing evidence that changes in the extent of immunoglobulin glycosylation can play an integral part in the pathogenesis of a number of immune-mediated inflammatory diseases [8]. We have shown that HIDS is associated with more extensive O-galactosylation of IgD and a reduction in O-sialylation of both IgD and IgA1 and that these changes are present both during acute febrile attacks and periods of quiescence.
The precise role of serum IgD in health and disease remains unclear making it difficult to judge the impact of these changes in IgD O-glycosylation in HIDS [13]. However, we do know that changes in IgA1 O-glycosylation can make a significant impact on IgA1 function [9]. In isolated human monocytes, IgD from patients with HIDS has been shown to be a potent inducer of a number of inflammatory cytokines including tumour necrosis factor alpha (TNF-α), IL-1β, IL-1 receptor antagonist, IL-6, IL-10 and leukemia inhibitory factor [14]. It has been suggested that uncontrolled secretion of these cytokines could lead to the intense inflammatory response characteristically seen in HIDS.
Activation of human monocytes by IgD is mediated through ill-defined IgD receptors (IgD-R) [13]. The best characterised IgD-Rs are those expressed by T lymphocytes [15]. These receptors preferentially bind IgD immune complexes, cross-linking by IgD leads to up-regulation of IgD-R and augmentation of antibody production, and significantly, these receptors are upregulated following vaccination [15–17]. Binding of IgD to murine and human T cell IgD-R is dependent on IgD glycosylation suggesting that the T cell IgD-R is a lectin [18]. In contrast to the murine IgD-R which binds IgD N-glycans, the human T cell IgD-R recognises the O-glycans of the IgD hinge region and is able to bind both IgD and IgA1 [19]. While IgA1 is capable of binding to the IgD-R it does not up-regulate IgD-R expression and effectively blocks activation of T cell IgD-R by IgD [19]. Clearly, changes in the O-glycosylation state of IgD and IgA1 could have a significant impact on affinity for the T cell IgD-R and the balance of activation and antagonism of IgD-R mediated T cell activation in HIDS. Any changes in this balance might be expected to make an impact on T cell orchestrated antibody production and could underlie the high basal levels of IgD and IgA1 seen in HIDS.
It has been noted for some time that there is no clear correlation between serum IgD (and IgA1) levels and the frequency or severity of febrile attacks, and it has been suggested that there might be an inhibitory compound that normally prevents IgD from eliciting an exaggerated cytokine response [14]. Up-regulation of the T cell IgD-R with vaccination may provide an alternative explanation. It is possible that the level of expression of IgD-Rs dictates the clinical course of febrile attacks and that this is determined by the balance between serum IgA1 glycoforms (blocking up-regulation of IgD-R) and IgD glycoforms (promoting IgD-R up-regulation), and concurrent external factors (such as vaccination) that are capable of independently up-regulating IgD-R expression. IgA1 and IgD levels in HIDS do not fluctuate in parallel, making the interplay between IgA1 and IgD glycoforms and IgD-R expression even more complex [4].
It is not known whether the myeloid IgD-R has similar properties to the T cell IgD-R but if it is also an O-glycan specific lectin then the observed changes in IgD and IgA1 O-glycosylation might also have significant effects on IgD-induced monocyte cytokine release. Further studies of the glycosylation of IgD and IgA1, and its impact on interactions with myeloid and T cell IgD-Rs will provide a better understanding of the pathogenesis of HIDS.
References
Drenth JP, van der Meer JW (2001) Hereditary periodic fever. N Engl J Med 345:1748–1757
Houten SM, Kuis W, Duran M, de Koning TJ, van Royen-Kerkhof A, Romeijn GJ, Frenkel J, Dorland L, de Barse MM, Huijbers WA, Rijkers GT, Waterham HR, Wanders RJ, Poll-The BT (1999) Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat Genet 22:175–177
Bodar EJ, van der Hilst JC, van Heerde W, van der Meer JW, Drenth JP, Simon A (2007) Defective apoptosis of peripheral-blood lymphocytes in hyper-IgD and periodic fever syndrome. Blood 109:2416–2418
Drenth JP, Haagsma CJ, van der Meer JW (1994) Hyperimmunoglobulinemia D and periodic fever syndrome. The clinical spectrum in a series of 50 patients. International Hyper-IgD Study Group. Medicine (Baltimore) 73:133–144
Klasen IS, Goertz JH, van de Wiel GA, Weemaes CM, van der Meer JW, Drenth JP (2001) Hyper-immunoglobulin A in the hyperimmunoglobulinemia D syndrome. Clin Diagn Lab Immunol 8:58–61
Ohtsubo K, Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126:855–867
Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA (2007) The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol 25:21–50
Alavi A, Axford JS (2008) Sweet and sour: the impact of sugars on disease. Rheumatology (Oxford) 47:760–770
Barratt J, Smith AC, Molyneux K, Feehally J (2007) Immunopathogenesis of IgAN. Semin Immunopathol 29:427–443
Smith AC, de Wolff JF, Molyneux K, Feehally J, Barratt J (2006) O-glycosylation of serum IgD in IgA nephropathy. J Am Soc Nephrol 17:1192–1199
Allen AC, Harper SJ, Feehally J (1995) Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol 100:470–474
Allen AC (1999) Methodological approaches to the analysis of IgA1 O-glycosylation in IgA nephropathy. J Nephrol 12:76–84
Preud’homme JL, Petit I, Barra A, Morel F, Lecron JC, Lelievre E (2000) Structural and functional properties of membrane and secreted IgD. Mol Immunol 37:871–887
Drenth JP, Goertz J, Daha MR, van der Meer JW (1996) Immunoglobulin D enhances the release of tumor necrosis factor-alpha, and interleukin-1 beta as well as interleukin-1 receptor antagonist from human mononuclear cells. Immunology 88:355–362
Coico RF, Tamma SL, Bessler M, Wei CF, Thorbecke GJ (1990) IgD-receptor-positive human T lymphocytes. I. Modulation of receptor expression by oligomeric IgD and lymphokines. J Immunol 145:3556–3561
Swenson CD, Cherniack EP, Russo C, Thorbecke GJ (1996) IgD-receptor up-regulation on human peripheral blood T cells in response to IgD in vitro or antigen in vivo correlates with the antibody response to influenza vaccination. Eur J Immunol 26:340–344
Swenson CD, Rizinashvili E, Amin AR, Thorbecke GJ (1995) Oligomeric IgD augments and monomeric IgD inhibits the generation of IgG memory antibody responses in normal, but not in IgD-deficient, mice. J Immunol 154:653–663
Amin AR, Tamma SM, Oppenheim JD, Finkelman FD, Kieda C, Coico RF, Thorbecke GJ (1991) Specificity of the murine IgD receptor on T cells is for N-linked glycans on IgD molecules. Proc Natl Acad Sci U S A 88:9238–9242
Swenson CD, Patel T, Parekh RB, Tamma SM, Coico RF, Thorbecke GJ, Amin AR (1998) Human T cell IgD receptors react with O-glycans on both human IgD and IgA1. Eur J Immunol 28:2366–2372
Acknowledgment
Anna Simon is supported by a grant from ZonMW VENI.
Conflict of interest statement
The authors declare that they have no conflict of interest related to the publication of this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
de Wolff, J.F., Dickinson, S.J., Smith, A.C. et al. Abnormal IgD and IgA1 O-glycosylation in hyperimmunoglobulinaemia D and periodic fever syndrome. Clin Exp Med 9, 291–296 (2009). https://doi.org/10.1007/s10238-009-0056-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-009-0056-y