Abstract
The interest in the properties of animal soft tissues is often related to the desire to find an animal model to replace human counterparts due to the unsteady availability of human tissues for experimental purposes. Once the most appropriate animal model is identified, it is possible to carry out ex-vivo and in-vivo studies for the repair of ligamentous tissues and performance testing of replacement and support healing devices. This work aims to present a systematic review of the mechanical properties of ligaments reported in the scientific literature by considering different anatomical regions in humans and several animal species. This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) method. Moreover, considering the lack of a standard protocol for preconditioning of tissues, this aspect is also addressed. Ninety-six studies were selected for the systematic review and analysed. The mechanical properties of different animal species are reported and summarised in tables. Only results from studies reporting the strain rate parameter were considered for comparison with human ligaments, as they were deemed more reliable. Elastic modulus, ultimate tensile stress, and ultimate strain properties are graphically reported identifying the range of values for each animal species and to facilitate comparison between values reported in the scientific literature in animal and human ligaments. Useful similarities between the mechanical properties of swine, cow, and rat and human ligaments have been found.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The interest in the mechanical properties of animal ligaments is often correlated with finding a useful model for human ones. Since ethical reasons make difficult to find human ligaments to run in vitro and in vivo tests, animal specimens are commonly employed. In fact, animal models are preferred in preclinical studies for two main types of research purposes: (i) evaluation of tissue healing through different strategies (for example, after growth factors and stem cell injection) and (ii) the evaluation of mechanical properties of suture pattern under validation and testing of innovative repair technologies. Surgical repair techniques commonly employed in human’s and animal’s traumatology (DeLong and Waterman 2015; Dabbene et al. 2018) rely on the results of mechanical studies, based on reported properties of the original and intact anatomical structures.
Nevertheless, not all animal ligaments are biomechanically comparable to their humans’ anatomical counterparts. Therefore, it is needed to discuss the differences between these latter and animal ligaments, even if few studies in the literature made a direct comparison between human and animal ligaments (Baah-Dwomoh et al. 2018; Noyes and Grood 1976).
This review aims to provide a more detailed analysis of similarities and differences between human and various animal species to find the most suitable human ligament surrogate. Uniaxial tensile tests performed on equine, bovine, ovine, caprine, swine, canine, rodents, leporidae, and human ligaments were considered. This work is closely related to a similar comparison between animal and human tendons previously conducted by this research group (Burgio et al. 2022).
1.1 Human and animal ligaments
Like tendons, ligaments are characterised by a hierarchical structure and are made of mesenchymal cells inside a supporting matrix and an extracellular matrix containing a high amount of collagen fibres (type I and type III collagen are the most abundant), water and to a lesser extent of elastin, glycoproteins, and proteoglycans (Rumian et al. 2007).
Despite the similar composition, in tendons collagen fibrils are placed in parallel to each other and along the whole length of the tendon. On the contrary, the collagen fibrils of the ligaments are not uniformly orientated, and this organisation is fundamental to withstand multidirectional loads (Rumian et al. 2007).
Even localisation and function, as well as the different arrangement of the components, contribute to defining differences in the biomechanical characteristics of tendons and ligaments: both of these structures must be able to withstand tensile loads, but while the tendons are subjected mostly to uniaxial forces, the ligaments are subjected to multiaxial loads (the force components directions depend on the directions of movement allowed to the joint) (Rumian et al. 2007).
1.2 Common applications of animal surrogates
Concerning biomechanics, it is important to consider that, unlike humans, almost all animals are quadrupeds and often have different and more limited ranges of motion in the corresponding joints (Bascuñán et al. 2019). However, there are many instances where they are extensively used. In this section, will be discussed different animal models encountered in the research. Considering human biomechanics, the main subject of investigation is the knee joint; therefore, over the years several studies with different animal models have been done to better understand its anatomy and biomechanics. On the other hand, only a few articles dealing with other anatomical sites were found, and these will be discussed in a specific subsection.
1.2.1 Animal models for knee joint
Knee joint ligaments injuries are one of the most widespread lesions; for this reason, several animal models have been widely employed to better understand the anatomy and biomechanics. Numerous studies dealing with knee ligament reconstruction via suture patterns, graft, or Ligament Advanced Reinforcement System (LARS) used animal specimens to perform tests, especially bovine (Eleswarapu et al. 2011), rabbit (Woo et al. 1992), rat (Yiannakopoulos et al. 2005), sheep (Weiler et al. 2001; Viateau et al. 2013), swine (Kim et al. 2014), and monkey (Noyes and Grood 1976). To the best of our knowledge, the study carried out by Noyes and Grood (Noyes and Grood 1976) is the only one in the literature that deals with a nonquadruped animal model, and the authors reported similar results with respect to the canine model.
The anterior cruciate ligament (ACL) is critical for knee joint stability in humans and animals, and its injury results in joint instability rapidly causing osteoarthritis (Comerford et al. 2005). The canine knee model is largely used to make studies on knee ligaments and tendons due to its similarity with its human counterpart (Beynnon et al. 1994).
The sheep stifle joint has often been used as an animal model for human ACL reconstruction. However, Radford et al. (1996), showed that the ovine stifle is not suitable for testing full-size human clinical ACL implants. The reason for this statement is that when compared to human joints the overall shape of the distal femur is narrower, and the femoral condyles do not have extensive articular surfaces distally. Thus, the range of motion of the stifle is not adapted for taking loads in full extension and cannot attain a straight-leg posture (Radford et al. 1996).
Moreover, it was concluded that the stifle joint of the sheep is both morphologically and biomechanically similar to the human knee, but there are detailed differences relating to ligament’s fibres geometry. In conclusion, the authors reported that the ovine stifle is a valid animal model for experimental work on menisci and cruciate ligaments (Radford et al. 1996).
The rabbit knee has often been used as an animal model for the study of cruciate ligaments (posterior cruciate ligament (PCL) and ACL) and collateral ligaments (medial collateral ligament (MCL) and the lateral collateral ligament (LCL)). It is well accepted in the orthopaedic community that unrepaired injuries to either cruciate ligament will eventually result in chronic secondary degenerative joint changes, most notably in the menisci and in the articular cartilage. Few studies have been proposed to analyse the pathological consequences of cruciate ligament ruptures in the medial and collateral ligaments. Among them, Tozilli and Arnoczky (Tozilli and Arnoczky 1988) have not found significant changes in the biomechanical properties of rabbit LCL after a complete section of the anterior and posterior cruciate ligaments.
Another knee ligament involved in common trauma is the MCL; therefore, it is of great importance to find suitable animal surrogates. A relevant case study was conducted by Germscheid et al. (2011), in which was reported that porcine MCL is comparable in shape and size and in its failure mechanism to the adult human MCL.
1.2.2 Other animal models
Animal models are often used also to investigate causes and consequences of human diseases on the related ligaments. For example, a frequent trauma highly explored is the chronic neck pain caused by whiplash; in this context, several tensile failure studies (Lee et al. 2006; Quinn and Winkelstein 2007) of the C6/C7 rat cervical facet capsular ligament have been conducted to better understand the whiplash-related pain. Other studies were also conducted to better understand pelvic floor disorders that often result on permanent compromission of pelvic ligaments, affecting millions of women every year. The pelvic anatomy of the Macaca species is approximately identical to that of the human, providing a unique opportunity to study pelvic supportive ligaments (Vardy et al. 2005) and related mechanical and structural changes after injuries. Studied on non-quadruped animals which have a certain relevance, since they have a posture and joint range of motion more similar to that of humans. Unfortunately, in our research work, only one study on non-quadrupeds animals met the eligibility criteria and therefore was considered worthy of being reviewed. The results obtained are interesting, and comparisons with human ligaments have been performed in paragraph 4.1.1.
1.3 Effects of experimental setup parameters
First of all, it is necessary to specify that to characterise the ligaments and evaluate the integrity of the tissues after surgical repair, uniaxial tensile tests are generally carried out on the bone–ligament–bone (blb) complexes rather than on the single, isolated ligament. This procedure is preferred due to the limited sizes of the single ligament and its slipperiness at the anchor points with the clamps. The bone provides a secure hold on clamps during in-vitro testing. In contrast, the blb complex has one drawback: often the break occurs near the insertions (avulsion) instead of the expected “mid-substance failure” (Sample 2017; Martin et al. 2015).
Due to the variability in the ligament’s mechanical properties introduced by the animal species, age, sex, testing conditions, tensile testing device and orientation of the ligaments or blb complexes in relation to the imposed stress, it is crucial to standardise a protocol to obtain data easily comparable with each other (Beynnon and Amis 1998). In this systematic review, wherever available, these parameters are always reported for completeness and proper comparison of the results. Nevertheless, this investigation of the existing scientific literature highlighted the lack of a commonly accepted standard. This point will be addressed in a dedicated section.
For example, there has been much discussion on the influence that the storage of the samples could have on the mechanical properties of the specimens. The debate is still open, but it seems that freezing up to three months does not significantly modify the structural and mechanical properties of the samples, as proven by Woo and colleagues (Woo et al. 1986), studying the influence of conservation on rabbit MCL ligaments (Martin et al. 2015; Beynnon and Amis 1998). In fact, in the main part of the experimental studies reported in this review, the specimens were kept at low temperature (freezing) and defrosted shortly before the actual test. Generally, specimens were maintained hydrated in solution during tests. For the conservation of the specimens, a physiological solution is commonly used, but also the phosphate buffered saline and Ringer's solution are usable (Martin et al. 2015).
The aim of this study is to analyse the setup parameters used during the experimental tests. In particular, two main factors influence the mechanical response: (i) the strain rate and displacement rate values set during the test and (ii) the preconditioning before the test. These aspects will be discussed in detail in the rest of the paper.
1.4 Difference between human and animals knee biomechanics
The substantial impact of knee ligaments injury, such as ACL, PCL, and collateral ligaments, has generated a big research field, thus allowing to explore their mechanisms of injury and the development of new treatment strategies. In fact, several large animal models are commonly used to study knee ligaments repair mechanisms, but no species is currently considered as the gold standard. However, each animal model has limitations, which should be carefully considered. Regarding the human ACL, it is well known that is anatomically divided into three bundles: the anteromedial (AM), intermediate (IM), and posterolateral (PL), each of them performing different functions within the knee joint. Other animal species as dog and goat ACL have only two bundles, rabbit ACL has not bundles, and only pig and goat ACL have three bundles (Bascuñán et al. 2019). Furthermore, biomechanical studies on the human ACL have shown that different bundles of ligaments have opposite behaviour during knee joint extension and flexion. Nevertheless, no animal ACL presents that mechanical behaviour in different portions (Bascuñán et al. 2019). Goat and swine appear to be a valid surrogate of ACL, since they present the greatest similarities with human ones (Bascuñán et al. 2019).
Another aspect to consider when experimental studies on knee animal models are designed is the difference in the mechanical properties of the knee ligaments at different angles of work. Wingfield et al. (2000) analysed the influence of two different knee angles in the mechanical properties of dog CraCl. However, no significant difference in the mechanical properties was found, but it is well known that cruciate ligaments in humans are influenced by the knee angle. Further studies need to evaluate more precisely this aspect.
2 Materials and methods
2.1 Eligibility criteria
The primary aim of this review is a systematic revision of the scientific literature reporting tensile-testing mechanical properties of healthy ligaments in different animal species (bovine, dog, equine, monkey, mouse, ovine, rabbit, rat, swine). The mechanical properties were collected to compare the mechanical behaviour and identify the most suitable animal model.
In the cases where the data were expressed in units of measures that did not belong to SI units, they were converted into the corresponding SI units. Furthermore, to improve data accuracy, the expression of these properties as mean value ± standard deviation (SD) was required. All articles that presented the following characteristics were excluded: (i) results of the tensile test represented only in a graphic form, expressed only as mean without standard deviation, percentage, or range of values; (ii) studies on pathological or damaged ligaments only; (iii) study conducted on ligaments harvested from paediatric or elderly patients; (iv) studies evaluating the healing process of injured ligaments through the insertion of allografts or autografts or that included the use of different kinds of scaffolds or growth factors; (v) studies that report only compression and shear stress values and viscoelastic properties of the specimens; (vi) studies with data derived from finite element models; (vii) studies that perform biaxial test.
2.2 Information sources and search
The main databases were PubMed, Google Scholar, Science Direct, Springer, Taylor and Francis, Wiley-Blackwell, and PicoPolito (Politecnico di Torino search engine). The keywords used to find the articles in the primary research were: “ligaments”, “animal ligaments”, “human ligaments”, “biomechanics”, “mechanical characterisation”, “mechanical properties”, “structural properties”, “stress–strain”, “tensile test”, “failure test”, “strain rate”, “Young’s modulus”, “ultimate tensile stress”, and “ultimate strain”. All the collected data were exported to Microsoft Excel and analysed. The research was conducted by four authors (S.C., M.M., F.S., and G.S.) working independently, each of them investigating one-quarter of the number of articles analysed and then reviewing them together one by one over three months. This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) method.
2.3 Data items
Specifically, the following mechanical properties were considered: elastic modulus or Young’s modulus (MPa), stiffness (\(N \, {\text{mm}}^{ - 1}\)), maximal load (N), ultimate tensile stress (MPa), ultimate strain (%), and energy absorbed at failure (\(N \, {\text{mm}}\)). Additionally, regarding the experimental setup of the tensile tests, the preconditioning application, the strain rate (\(\%\, {\text{min}}^{ - 1}\)), and the displacement rate (\({\text{mm\,min}}^{ - 1}\)) values set for the tests were reported.
2.4 Additional analysis
In order to evaluate all the aspects related to the experimental tensile tests, the two methodologies that are employed to perform the tests were considered: “strain-controlled mode” and “displacement-controlled mode”. The information about the control mode adopted by various authors during tensile tests was reported with the relative values of strain rate, where “SCM” stands for “strain controlled mode” and “DCM” stands for “displacement-controlled mode”.
Additionally, the type of preconditioning used for the tests was reported and evaluated to give some guidelines in the results section.
3 Results
3.1 Study selection
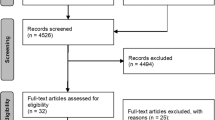
The initial research of peer-reviewed articles published in the selected databases using the mentioned keywords includes more than 2000 manuscripts. Then, the title and abstracts were analysed to include the papers and 263 manuscripts for the full-text evaluation were selected. Following the eligibility criteria, 95 articles were evaluated to obtain values of the mechanical properties (Fig. 1).
In particular, data were classified in animal species as follows: cow (n = 3), calf (n = 1), dog (n = 10), horse (n = 5), foal (n = 1), monkey (n = 3), mouse (n = 2), goat (n = 6), sheep (n = 10), rabbit (n = 12), rat (n = 7), swine (n = 7), and human (n = 29). We considered any peer-reviewed article published in English between 1968 and the current date (May 2022).
3.2 Synthesis of results
After selecting the articles that were in compliance with the eligibility criteria, all the data regarding ligament mechanical and the type of preconditioning used in the published studies were reported in many summary tables. Article summaries are illustrated in Table 1, grouped by animal species and human. Table 2 reports a list of the ligament acronyms as used in this paper.
3.3 Study characteristics
Table 3 shows the mechanical properties (strain rate and/or displacement rate, Young’s modulus, stiffness, maximum load, ultimate tensile stress, ultimate strain, and energy absorbed at failure) in different animal species considering the control mode (only in the studies in which strain rate was used) and preconditioning. Table 4 reports the same mechanical values for different human ligaments.
3.4 Comparison between the mechanical properties of animal and human ligaments
All the collected data reported in the previous tables were organised in different bar graphs. Each bar in the graphs represents the range of values assumed by a specific mechanical property analysed; the bar is delimited by the standard deviation (STD) values centred on the mean value of the data considered. In certain cases, the same reference provides several bars with different values because, in the same article, animals of different breeds, different sexes, different ages, or right/left limbs were studied. As a result, different values were obtained in the same article, although the type of sample preparation and strain/displacement rate were the same.
All the data reported in the previous tables were organised in different bar graphs. The elastic modulus, the ultimate tensile stress, and the ultimate strain report the strain rate in mm/min (Figs. 2, 3 and 4) and in %/min (Figs. 5, 6 and 7). For standardisation, values reported in mm/min and in cm/min have been modified to obtain values in mm/s. Data that did not report the strain rate values were not used for graphing and analysis. During the evaluation of all the articles related to rabbit ligaments, different MCL elastic modulus values were found. In particular, the article of Xie et al. (Xie et al. 2021) shows an MCl elastic modulus equal to 3 GPa, a greater value compared to the other articles. The high variability in the results may be due to the experimental setup, since they used a tension–torsion combined testing machine. Given that the elastic modulus value obtained by Xie et al. appears to be an outlier, this study was removed from our evaluation.
For better data visualisation and comparison of the mechanical properties of the ligaments between different animal species and the human, each species was associated with a specific colour: bovine (blue), dog (light blue), equine (green), monkey (light green), goat (yellow), sheep (orange), rabbit (red), rat (fuchsia). Regarding the mechanical properties of the human ligaments, grey was chosen.
3.5 Results of mechanical property evaluation in mm/min
3.6 Results of mechanical property evaluation in %/min
3.7 Results of additional analysis-type of preconditioning
Table 5 reports the preconditioning that has been performed for different animal species and human.
4 Discussion
The mechanical properties evaluation of animal’s and human’s ligaments obtained from literature was performed in this review, considering the strain rate with two different units (mm/min and %/min). The analysis only dealt with the comparison between human and animal ligaments; thus, no comparison was performed among the mechanical properties of animal ligaments. From the analysis of the bar graphs, it was observed that generally, for each species, the values of the mechanical properties are included in a specific range. In particular, there is evidence that the value of strain rate has an effect on the mechanical properties of the ligaments (Pioletti et al. 1999). Differences in specimen behaviour at high and low strain rate values were shown in several papers. For instance, (Woo et al. 1990a) showed that the rabbit MCL ligament changes its properties at high strain rate values compared to low strain rate values (Figs. 5, 6 and 7). In other cases, for the same strain rate values, some mechanical properties show very different value as data obtained for rabbit MCL, v = 10 mm/min (Weiss et al. 1991) for elastic modulus (Fig. 2). Before the evaluation of the similarity between human ligaments and animal ligaments, it is important to specify that two different types of overlapping were found. The partial similarity means an overlapping between data, but the animal ligament shows a range of values that exceed human ligament values range. On the other hand, total similarity means that the animal ligaments show a range of values that is within the human ligament values range. The partial and total similarity between human and animal ligaments is reported in Appendix 1 and 2 in Supplementary material. Only the total similarity for all the parameters evaluated in this work is discussed in the following subsection, additionally, the percentage of overlap between the animal species and human ligament range was reported (%, of overlap between the distributions considered as the overlap with respect to the human values range).
4.1 Evaluation of mechanical property
4.1.1 Evaluation of mechanical property in mm/min
Analysing the mechanical parameters obtained with a strain rate in mm/min (as reported in Figs. 2, 3 and 4), it can be observed that:
-
Human AL (Zens et al. 2015) has a partial similarity for each animal ligament in terms of elastic modulus and ultimate stress. It has a verified total similarity of 38,7% in terms of ultimate strain with dog CraCL (Wingfield et al. 2000).
-
Human AB-IGHL (Moore et al. 2005) has a total similarity with the swine CL (left) (Tan et al. 2015). This surrogate presents an error with respect to the human equal to 11.06% for elastic modulus, 52.50% for ultimate stress, and 58.89% for ultimate strain. Considering the elastic modulus, there are other total similarities: 17.09% with swine CL (right) (Tan et al. 2015) and 4.33% with sheep ACL (Gurlek et al. 2017). The ultimate stress presents only partial similarities. The ultimate strain presents total similarities with swine USL (Tan et al. 2015) of 24.57%, swine CL (right) of 70.33%, swine MCL (Germscheid et al. 2011) between 10.16% and 12.71%, swine posterolateral ACL (Zhou et al. 2009) of 42.37%, swine PCL of 20.76% and ACL between 19.91% and 21.61% (Hirokawa and Sakoshita 2003), and dog CraCL (Wingfield et al. 2000) between 7.33% and 26.52%.
-
Human PB-IGHL (Moore et al. 2005) presents total similarities in terms of elastic modulus with swine USL of 58.09% (Tan et al. 2015). For the ultimate strain, there are total similarities with swine USL (Tan et al. 2015) of 52.25%, swine MCL (Germscheid et al. 2011) between 21.62% and 27.02%, swine ACL of 42.34% (Hirokawa and Sakoshita 2003), rat MCL (Su et al. 2008) between 25.22% and 30.63%, rabbit MCL (Weiss et al. 1991) between 4.50% and 6.30%, rabbit MCL (Woo et al. 1992) of 9.00%, and rabbit (female, 36 and 12 months) between 4.50% and 11.71% (Woo et al. 1990c).
-
Human RL (Martins et al. 2013) has only partial similarities for all the parameters.
-
Human ALL/PLL (mean) (Przybylski et al. 1996) presents total similarities in terms of elastic modulus with swine USL) (Tan et al. 2015) of 12.29%, dog ACL (Comerford et al. 2005) between 12.00% and 18,83%, swine ACL of 34.00%, and PCL of 19.33% (Hirokawa and Sakoshita 2003).
-
Human USL (Martins et al. 2013) shows no similarities.
-
Human IGHL (older) (Lee et al. 1999) presents a total similarity in terms of elastic modulus with dog ACL (Comerford et al. 2005) of 72.72%. For the ultimate stress, there are only partial similarities. For the ultimate strain, there is a total similarity with rabbit MCL (male,12 months) of 80% (Woo et al. 1990c).
-
Human IGHL (younger) (Lee et al. 1999) presents only partial similarities for elastic modulus and ultimate strain.
-
Human PF (Pieroh et al. 2016) presents a total similarity in terms of elastic modulus with dog ACL (Comerford et al. 2005) of 22.42%. For the ultimate strain, there are only partial similarities.
-
Human IS (Pieroh et al. 2016) presents a total similarity in terms of elastic modulus with swine USL (Tan et al. 2015) of 36.16%. For the ultimate strain, there are only partial similarities.
-
Human IL (Pieroh et al. 2016) presents a total similarity in terms of elastic modulus with swine USL (Tan et al. 2015) of 34.47%. For the ultimate strain, there are only partial similarities.
-
Human PF (Schleifenbaum et al. 2016) presents a total similarity for elastic modulus of swine USL (Tan et al. 2015) of 26.49%. For the ultimate strain, there are total similarities with swine CL(left) (Tan et al. 2015) of 53.29%, swine posterolateral ACL (Zhou et al. 2009) of 37.86%, sheep ACL (right and left) (Rogers et al. 1990) between 44.49% and 71.94%, monkey ACL (Noyes and Grood 1976b) between 58.68% and 63.89%, and dog ACL (Comerford et al. 2005) between 46.00% and 52.06%.
-
Human IS (Schleifenbaum et al. 2016) presents a total similarity in terms of elastic modulus with swine USL (Tan et al. 2015) of 34.96%. For the ultimate strain, there are total similarities with sheep ACL (right and left) (Rogers et al. 1990) between 16.66% and 20.00%, dog ACL (Figgie et al. 1986) between 3.33% and 20.00%, and dog ACL (Shino et al. 1984) between 26.00% and 36.00%.
-
Human IL (Schleifenbaum et al. 2016) presents a total similarity in terms of elastic modulus with swine USL (Tan et al. 2015) of 35.13%. For the ultimate strain, there are total similarities with sheep ACL (right and left) (Rogers et al. 1990) between 13.88% and 16.66%, dog ACL (Figgie et al. 1986) between 2.77% and 16.66%, and dog ACL (Shino et al. 1984) between 21.66% and 30.00%.
-
Human posterior MFL (Gupte et al. 2002) presents total similarities in terms of elastic modulus with dog CraCL (Wingfield et al. 2000) between 7.89% and 28.19%, sheep ACL (Meller et al. 2008) of 45.41%, sheep ACL (right and left) (Rogers et al. 1990) between 15.68% and 39.21%, rat MCL (Su et al. 2008) of 35.29%, and swine ACL of 23.45% and PCL of 27.45% (Hirokawa and Sakoshita 2003).
-
Human anterior MFL (Gupte et al. 2002) presents total similarities in terms of elastic modulus with sheep ACL (Meller et al. 2008) of 24.16%, sheep ACL (right and left) (Rogers et al. 1990) between 8.34% and 20.86%, rat MCL (Su et al. 2008) between 17.94% and 18.78%, swine MCL (Germscheid et al. 2011) between 19.82% and 45.86%, swine ACL (Zhou et al. 2009) of 25.77%, swine posterolateral ACL (Zhou et al. 2009) of 19.01%, swine anteromedial ACL (Zhou et al. 2009) of 12.55%, swine PCL between 4.86% and 14,60%, and ACL between 8.51% and 12.47% (Hirokawa and Sakoshita 2003).
-
Human MFL (Kusayama et al. 1994a) presents total similarities in terms of elastic modulus with dog CraCL (Wingfield et al. 2000) between 25.64% and 55.55%, sheep ACL (Meller et al. 2008) of 21.36%, sheep ACL (right and left) (Rogers et al. 1990) between 4.29% and 15.36%, rabbit MCL (Woo et al. 1992) medial of 51.28% and lateral of 67.52%, rat MCL (Su et al. 2008) between 38.46% and 47.00%, swine MCL (Germscheid et al. 2011) between 18.37% and 29.48%, and swine ACL of 23.24% and PCL of 46.96% (Hirokawa and Sakoshita 2003).
-
Human PFL (LaPrade et al. 2005) presents a total similarity in terms of elastic modulus with swine USL (Tan et al. 2015) of 50.88%.
-
Human antero-lateral PCL (Race and Amis 1994) presents total similarities in terms of elastic modulus with dog CraCL (Wingfield et al. 2000) between 8.45% and 30.21%, rat MCL (Su et al. 2008) between 36.16% and 37.81%, sheep ACL (right and left) (Rogers et al. 1990) between 16.80% and 42.01%, and swine ACL of 25.12% and PCL of 29.41% (Hirokawa and Sakoshita 2003). For the ultimate stress, there are total similarities with dog ACL (Comerford et al. 2005) between 20.39% and 23,02%, sheep ACL (Hunt et al. 2005) of 29.60%, sheep ACL (Weiler et al. 2004) of 29.60%, rat MCL (Su et al. 2008) of 58.55%, and swine PCL (Hirokawa and Sakoshita 2003) of 27.63%.
-
Human postero-medial PCL (Race and Amis 1994) presents total similarities in terms of elastic modulus with dog CraCL (Wingfield et al. 2000) of 14.57%, swine ACL (Zhou et al. 2009) of 89.49%, swine posterolateral ACL (Zhou et al. 2009) of 66.01%, swine anteromedial ACL (Zhou et al. 2009) of 43.60%, and swine ACL (Hirokawa and Sakoshita 2003) of 29.56%. For the ultimate stress, there are total similarities with swine ACL between 36.00% and 53.00%, PCL of 32.00% (Hirokawa and Sakoshita 2003), and swine posterolateral ACL (Zhou et al. 2009) of 66.60%.
-
Human Cal (older) (Fremerey et al. 2000) presents total similarities in terms of ultimate stress with dog ACL (Comerford et al. 2005) of 36.47%, sheep ACL (Gurlek et al. 2017) of 27.53%, and swine PCL (Hirokawa and Sakoshita 2003) of 49.41%. For the ultimate strain, there are total similarities with swine USL (Tan et al. 2015) of 67.44%, and rat MCL (Su et al. 2008) between 32.55% and 39.53%.
-
Human Cal (younger) (Fremerey et al. 2000) presents total similarities in terms of ultimate stress with dog ACL (Comerford et al. 2005) of 40.78%, sheep ACL (Gurlek et al. 2017) of 30.78%, and swine PCL (Hirokawa and Sakoshita 2003) of 55.26%. For the ultimate strain, there are total similarities with swine MCL (Germscheid et al. 2011) between 40.67% and 50.84%.
-
Human AB-IGHL/PB-IGHL/SB-IGHL (mean) (Bigliani et al. 1992) presents total similarities in terms of ultimate strain with swine MCL (Germscheid et al. 2011) between 31.46% and 33.70%, and rat MCL (Su et al. 2008) of 38.20%.
-
Human FAL (Hewitt et al. 2002) presents total similarities in terms of ultimate strain with swine MCL (Germscheid et al. 2011) between 32.00% and 40.00%, swine ACL of 62.66% and PCL of 33.33% (Hirokawa and Sakoshita 2003), rat MCL (Su et al. 2008) of 37.33%, rabbit MCL (Moon et al. 2006) of 37.33%, rabbit MCL (Weiss et al. 1991) between 6.66% and 10.66%, rabbit MCL (Woo et al. 1992) of 13.33%, rabbit female (from 6 to 36 months) between 6.66% and 17.33% and rabbit male (from 6 to 36 months) between 10.66% and 28.00% (Woo et al. 1990c).
-
Human IHIL (Hewitt et al. 2002) presents total similarities in terms of ultimate strain with swine PCL of 50.00% (Hirokawa and Sakoshita 2003), rabbit MCL (Moon et al. 2006) of 56.00%, rabbit MCL (Weiss et al. 1991) between 10.00% and 14.00%, and rabbit female (from 6 to 36 months) between 10.26% and 26.00% and rabbit male (from 6 to 36 months) between 16.00% and 42.00% (Woo et al. 1990c).
-
Human IS (Hewitt et al. 2002) presents total similarities in terms of ultimate stress with monkey RL (Vardy et al. 2005) of 65.08%, and swine CL of 28.99% (right) and 12.42% (left) (Tan et al. 2015).
-
Human SHIL (Hewitt et al. 2002) presents total similarity in terms of ultimate stress with swine USL of 28.94% (Tan et al. 2015), and swine DL (Polak et al. 2014) of 50.00%.
-
Human Scapholunate Ligament presents total similarities in terms of ultimate strain with sheep ACL (right and left) (Rogers et al. 1990) between 41.32% and 49.58%, and dog ACL (Figgie et al. 1986) of 8.26%.
-
Human PFL (Sugita and Amis 2001) presents total similarities in terms of ultimate strain with swine MCL (Germscheid et al. 2011) between 46.15% and 57.69%, swine PCL of 90.38% (Hirokawa and Sakoshita 2003), rat MCL (Su et al. 2008) of 53.84%, rabbit MCL (Woo et al. 1992) of 19.23%, rabbit MCL (Weiss et al. 1991) between 9.61% and 13.46%, rabbit female (12 months) of 9.61% and rabbit male (36 months) of 23.07% (Woo et al. 1990c).
-
Human LCL (Sugita and Amis 2001) presents total similarities in terms of ultimate strain with rabbit MCL (Woo et al. 1992) of 40.00%, and rabbit MCL (Weiss et al. 1991) of 20%.
4.1.2 Evaluation of mechanical property in %/min
Analysing the mechanical parameters obtained with a strain rate in %/min in Figs. 5, 6 and 7, it can be observed that:
-
Human ACL (Noyes and Grood 1976) has no similarities for elastic modulus. For the ultimate stress, there are only partial similarities with calf CauCL, LCL and MCL (Eleswarapu et al. 2011).
-
Human ACL (Chandrashekar et al. 2006) has only partial similarities for elastic modulus and ultimate stress. Instead, for the ultimate strain, there are total similarities with goat ACL (Jackson et al. 1991) between 25% and 33.33%.
-
Human anterolater PCL (Race and Amis 1994) presents total similarities in terms of elastic modulus with monkey ACL (Noyes and Grood 1976) of 21.82% and goat ACL (Jackson et al. 1993) of 31.09%. For the ultimate stress, there is total similarities with swine LCL (Bonner et al. 2015) of 72.36%. For the ultimate strain, there is a total similarity with swine LCL (Bonner et al. 2015) of 56.60%.
-
Human posteromedial PCL (Race and Amis 1994) presents total similarities in terms of elastic modulus with monkey ACL (Noyes and Grood 1976) of 37.68% and sheep ACL (Mahalingam et al. 2015) of 46.37%. For the ultimate strain, there are total similarities with swine LCL (Bonner et al. 2015) of 55.55%, and with equine SL (Smith 2006) of 96.29%.
-
Human FCL (LaPrade et al. 2005) presents total similarities in terms of elastic modulus with monkey ACL (Noyes and Grood 1976) of 23.48% and sheep ACL (Mahalingam et al. 2015) of 28.90%.
-
Human MCL (longitudinal) (Quapp and Weiss 1997) presents only partial similarities for elastic modulus and ultimate strain.
-
Human MCL (transverse) (Quapp and Weiss 1997) presents total similarities in terms of elastic modulus with cow PL (Oskui et al. 2016) at different strain rate values, 0.28% (600%/min), 7.56% (6000%/min), and 9.80% (60,000%/min).
-
Human MPFL (Criscenti et al. 2016) presents total similarity in terms of ultimate stress with calf MCL (Eleswarapu et al. 2011). For ultimate strain, there is a total similarity with goat ACL (Jackson et al. 1991) of 29.48%.
-
Human ALL (strain rate of 3000%/min) (Mattucci et al. 2012) presents a total similarity in terms of ultimate strain with cow PL (Oskui et al. 2016) at strain rate of 6000%/min and 60,000%/min of 6.12%.
-
Human ALL (strain rate of 12,000%/min) (Mattucci et al. 2012) presents only partial similarities for ultimate stress.
-
Human ALL (strain rate of 900,000%/min) (Mattucci et al. 2012) presents only partial similarities for ultimate stress.
-
Human PLL (strain rate of 3000%/min) (Mattucci et al. 2012) presents only partial similarities for ultimate stress.
-
Human PLL (strain rate of 12,000%/min) (Mattucci et al. 2012) presents total similarities in terms of ultimate stress with PL (Oskui et al. 2016) of 0.44% (60%/min), 0.82% (600%/min), 0.98% (6000%/min), 1.45% (60,000%/min), with calf CraCL of 1.9%, CauCL of 18.70%, LCL of 12.36%, MCL of 20.28% (Eleswarapu et al. 2011), and swine LCL (Bonner et al. 2015) of 34.86%. For the ultimate strain, there are a total similarities with dog CraCL (Butler et al. 1983) of 5.31%, equine SL (Riemersma and Schamhardt 1985) of 1.27%, equine SL of 2.97% and DCL of 2.12% (Jansen and Savelberg 1994), equine SL (Smith 2006) of 11.06%, equine AccL (Becker et al. 1994) between 4.25% and 6.38%, goat ACL (Jackson et al. 1991) between 4.25% and 8.50%, rabbit MCL (Woo et al. 1990a) between 1.06% and 2.12%, rabbit MCL (Moon et al. 2006) of 5.95%, and swine LCL (Bonner et al. 2015) between 4.25% and 6.38%.
-
Human PLL (strain rate of 900,000%/min) (Mattucci et al. 2012) presents a total similarity in terms of elastic modulus with sheep ACL (Mahalingam et al. 2015) of 46.37%. For ultimate stress, there is a total similarity with swine LCL (Bonner et al. 2015) of 72.36%.
-
Human CL (strain rate of 3000%/min) (Mattucci et al. 2012) presents total similarities in terms of elastic modulus with cow PL (Oskui et al. 2016) at different strain rate values, of 1.25% (60%/min), 0.31% (600%/min). For ultimate stress, there are similarities with cow PL (Oskui et al. 2016) at different strain rate values, of 11.66% (60%/min), 21.66% (600%/min), 25.83% (6000%/min), and 38.33% (60,000%/min).
-
Human CL (strain rate of 12,000%/min) (Mattucci et al. 2012) presents total similarities in terms of elastic modulus with cow PL (Oskui et al. 2016) at different strain rate, of 0.29% (600%/min), 7.94% (6000%/min). There are only partial similarities for ultimate strain with cow PL (Oskui et al. 2016) with strain rate of 600, 6000 and 60,000%/min.
-
Human CL (strain rate of 900,000%/min) (Mattucci et al. 2012) presents total similarities in terms of elastic modulus with cow PL (Oskui et al. 2016) at different strain rate values, of 0.29% (600%/min), 7.94% (6000%/min), and 7.94% (60,000%/min).
-
Human LF (strain rate of 3000%/min) (Mattucci et al. 2012) presents total similarities in terms of elastic modulus with cow PL (Oskui et al. 2016) at different strain rate, of 1.79% (6000%/min), 2.31% (60,000%/min), and calf LCL (Eleswarapu et al. 2011) of 26.95%.
-
Human LF (strain rate of 12,000%/min) (Mattucci et al. 2012) presents only partial similarities for elastic modulus and ultimate stress.
-
Human LF (strain rate of 900,000%/min) (Mattucci et al. 2012) presents only partial similarities for elastic modulus and ultimate stress.
-
Human ISL (strain rate of 3000%/min) (Mattucci et al. 2012) presents total similarities in terms of elastic modulus with calf LCL of 40.7%, CauCL of 59% (Eleswarapu et al. 2011), and with cow PL (Oskui et al. 2016) at different strain rate, of 0.40% (60%/min), 0.1% (600%/min), 2.70% (6000%/min) and 3.50% (60,000%/min). For ultimate stress, there are total similarities with cow PL (Oskui et al. 2016) at different strain rate values, of 4.82% (60%/min), 8.96% (600%/min), 10.69% (6000%/min), and 15.86% (60,000%/min).
-
Human ISL (strain rate of 12,000%/min) (Mattucci et al. 2012) presents total similarities in terms of elastic modulus with calf CauCL of 90.77% (Eleswarapu et al. 2011), and with cow PL (Oskui et al. 2016) at different strain rate, of 2.15% (60%/min), 4.00% (600%/min), 4.76% (6000%/min) and 7.36% (60,000%/min). For ultimate stress, there are total similarities with calf LCL of 90.76%, CauCL of 59% (Eleswarapu et al. 2011), and with cow PL (Oskui et al. 2016) at different strain rate, of 2.15% (60%/min), 4.00% (600%/min), 4.76% (6000%/min), and 7.07% (60,000%/min). For the ultimate strain, there are total similarities with dog CraCL (Butler et al. 1983) of 20.83%, goat ACL (Jackson et al. 1991) between 16.66% and 33.33%.
-
Human ISL (strain rate of 900,000%/min) (Mattucci et al. 2012) presents total similarities in terms of ultimate stress with calf CauCL of 95.16% (Eleswarapu et al. 2011), and cow PL (Oskui et al. 2016) at different strain rate, of 2.25% (60%/min), 4.19% (600%/min), 5.00% (6000%/min), and 7.41% (60,000%/min). For the ultimate strain, there is a total similarity with dog CraCL (Butler et al. 1983) of 20.83%.
-
Human LF(Nachemson and Evans 1968), presents total similarities in terms of the ultimate stress with calf CraCL (Eleswarapu et al. 2011) from 32.42 to 66.66%, and cow PL (Oskui et al. 2016) at different strain rate, of 12.72% (60%/min), 23.63% (600%/min), and 41.81% (60,000%/min). For ultimate strain, specimens with an average ultimate stress of 21.60 MPa have a total similarity with equine SL (Smith 2006) of 81.25%.
4.2 Type of preconditioning
The preconditioning consists typically of 10/20 cycles of loading/unloading until a certain value or inside an interval of tension or deformation. As can be seen in Table 5, in the majority of the reviewed articles, the specimens underwent preconditioning by 10 cycles of approximately 0–5% strain (Shetye et al. 2009, Woo et al. 1990b, Ng et al. 1995, Woo et al. 1992, Danto and Woo 1993, Murao et al. 1997, Ma et al. 2009, Moon et al. 2006, Kim et al. 2014, Wilson et al. 2012, Quapp and Weiss 1997, Criscenti et al. 2016, Kusayama et al. 1994, Hewitt et al. 2002, Schleifenbaum et al. 2016, Moore et al. 2004 and Moore et al. 2005) or around 50 N (Becker et al. 1994, Viateau et al. 2013, Wijdicks et al. 2010 and Robinson et al. 2005). It is also possible to observe that in many cases (Diotalevi et al. 2018, Woo et al. 1990b, Abramowitch et al. 2003, Weiler et al. 2004, Woo et al. 1992, Panjabi et al. 1996, Woo et al. 1986, Weiss et al. 1991, Xie et al. 2021, Kim et al. 2014, Tan et al. 2015 and Schleifenbaum et al. 2016) the loading/unloading cycles are performed at the same strain rate used during the tensile tests. Lastly, it can be said that the type of preconditioning varies with different ligaments in various animal species and human specimens. In fact, it is important to point out that in general there is no standardisation in terms of the number of cycles and the value of deformation or tension at which the preconditioning is performed.
4.3 Limitations
The individuation from the existing scientific literature of the most suitable surrogate to imitate the behaviour of human ligaments is hampered by several inhomogeneities in the experimental test protocol. This study also did not consider parameters such as animal age, sex, and lifetime activity. These parameters may influence the biomechanical characteristics of soft tissues. Additionally, the comparison of ligaments should be conducted by evaluating their composition. Future studies should compare the influence of these parameters on the mechanical properties of animal and human tendons, which would lead to a more accurate assessment of the ligament to be used for ex vivo testing. Moreover, here the mechanical properties of knee animals and human ligaments were reported evaluating only a uniaxial tensile test condition. Further studies will be needed to analyse their mechanical behaviour at different angles.
5 Conclusions
This systematic review aimed at defining the most suitable surrogates for mimicking the behaviour of human ligaments when subjected to uniaxial tensile tests. For this reason, the scientific literature was reviewed, evaluating the experimental studies involving the mechanical properties of animal ligaments. Differences and similarities between human and animal ligaments were highlighted and commented upon and the best candidates were determined and discussed. The comparison between the mechanical properties of animal ligaments highlighted how they cannot always be compared with their human counterparts; on the other hand, there are many similarities between different anatomical parts. In general, no specific animal ligaments can provide a suitable model for its respective human counterpart concerning all the three primary mechanical properties (Young modulus, ultimate tensile stress, and ultimate tensile strain) at the same strain rate. It is interesting to note that in the current scientific literature, different animal models (bovine, dog, rabbit, and swine) were adopted to evaluate the knee repair technologies; nevertheless, despite this wide use, no clear similarities were found in their mechanical properties. Further studies will be needed to further compare the mechanical properties of these ligaments and ensure that the scientific evidence derived from such experimental studies can be considered reliable.
Several similarities were observed in some properties between animal and human ligaments. These similarities were found despite the ligaments having been analysed at different strain rates. The results showed similarities between animal and human ligaments that should be considered in the evaluation of scaffolds and sutures.
Considering the results reported for tests performed in mm/min:
-
Swine CL with a displacement rate of 45 mm/min is comparable (total similarity in terms of elastic modulus, ultimate tensile stress and ultimate strain) with human AB-IGHL with a displacement rate of 10 mm/min;
-
Swine USL with a displacement rate of 45 mm/min is comparable (total similarity in terms of elastic modulus and ultimate strain but not for ultimate stress) with human PB-IGHL with a displacement rate of 10 mm/min;
-
Swine ACL and posterolateral ACL with a displacement rate of 19.8 mm/min are comparable (total similarity in terms of elastic modulus and ultimate strain but not for ultimate stress) with human posteromedial PCL with a displacement rate of 1000 mm/min;
-
Rat MCL with a displacement rate of 30 mm/min is comparable (total similarity in terms of elastic modulus and ultimate stress but not for ultimate strain) with human posteromedial PCL with a displacement rate of 1000 mm/min;
-
Swine PCL with a displacement rate of 19.8 mm/min is comparable (total similarity in terms of elastic modulus and ultimate stress but not for ultimate strain) with human anterolateral PCL with a displacement rate of 1000 mm/min;
It’s important mentioning that monkey RL with a displacement rate of 6 mm/min has a partial similarity with human RL with a displacement rate of 5 mm/min for elastic modulus and ultimate tensile stress. This result should be further analysed in future works.
Considering the results reported for tests performed in %/min:
-
Swine LCL with a strain rate of 60%/min is comparable (total similarity in terms of ultimate stress and ultimate strain but not for elastic modulus) with human anterolateral PLL with a strain rate of 12,000%/min;
-
Swine LCL with a strain rate of 6o %/min and 600%/min are comparable (total similarity in terms of ultimate stress and ultimate strain but not for elastic modulus) with human anterolateral PCL with a strain rate of 3000%/min;
-
Swine LCL with a strain rate of 6o %/min is comparable (total similarity in terms of ultimate stress and ultimate strain but not for elastic modulus) with human PLL with a strain rate of 12,000%/min;
-
Cow PL with a strain rate of 6o %/min and 600% is comparable (total similarity in terms of elastic modulus and ultimate stress but not for ultimate strain) with human CL with a strain rate of 3000%/min. Moreover, the cow PL at different strain rate shows some partial similarities with human CL with a strain rate of 900,000%/min;
-
Cow PL with a different strain rate is comparable (total similarity in terms of elastic modulus and ultimate stress but not for ultimate strain) with human ISL with strain rates of 3000%/min and 12,000%/min. The human ISL (3000%/min and 12,000%/min) shows some partial similarities with calf CauCL for elastic modulus and ultimate stress. Moreover, increasing the strain rate, some partial similarities with cow PL remain.
In our previous review, similarities between human, swine, equine, rabbit, rat, and goat tendons were found and discussed in detail. Here, the analysis of the mechanical properties for human and animal ligaments reported similarities between human and swine, cow, and rat ones. Comparing these two reviews, it can be stated that there are similarities between the mechanical properties of human and animals’ tendons and ligaments. In particular, the species with most similarities for both tendons and ligaments are swine and rat. These results may pave the way for future works.
As a concluding remark, it seems highly probable that the choice of parameter setting significantly affects the results of the experimental studies reviewed and discussed here. Unfortunately, different authors reported their results with different settings. The lack of standard test settings (strain rate, pre-conditioning) for the experiments should be considered when interpreting the results reported in the scientific literature. Future studies will be needed to evaluate ligaments from different animals and anatomical regions with the same test conditions and strain rate, in a fully comparable way. Based on the evaluation of mechanical characterisation of ligaments analysed in this work, the authors thought the following suggestions for best practices. After the tendon extraction from the anatomical site, it is important to use the same protocol for each of them. It is advisable to not perform the test on frozen samples. However, in case of frozen samples, the defrosting process should be done at least 24 h before the tests. Furthermore, before the test, the specimens’ thickness and width should be measured. These measurements can be done either in a normal condition or with a preload. The preload value should be evaluated based on the literature information; if no data are available, the preload should not exceed 10 Newton. Of course, all the parameters used for the test should be fully reported in the article and defined after an evaluation of the literature on the specific tissue. Based on this review, the standard preconditioning for ligaments should be 20 cycles at 1%/s of strain rate (starting from the preload force). Finally, the range where Young’s modulus was calculated should be reported in the article.
References
Abramowitch SD, Papageorgiou CD, Debski RE et al (2003) A biomechanical and histological evaluation of the structure and function of the healing medial collateral ligament in a goat model. Knee Surg Sports Traumatol Arthrosc 11:155–162. https://doi.org/10.1007/s00167-002-0336-5
Baah-Dwomoh A, Alperin M, Cook M, De Vita R (2018) Mechanical analysis of the uterosacral ligament: swine vs. human. Ann Biomed Eng 46:2036–2047. https://doi.org/10.1007/s10439-018-2103-x
Bascuñán AL, Biedrzycki A, Banks SA et al (2019) Large animal models for anterior cruciate ligament research. Front Vet Sci. https://doi.org/10.3389/fvets.2019.00292
Becker CK, Savelberg HHCM, Barneveld A (1994) In vitro mechanical properties of the accessory ligament of the deep digital flexor tendon in horses in relation to age. Equine Vet J 26:454–459. https://doi.org/10.1111/j.2042-3306.1994.tb04049.x
Belanger M, Moore D, McAlistair S, Ehrlich M (2000) The mechanical properties of rat ACL are independent of serum estrogen level. In: Session 26-anterior cruciate ligament-VALENCIA B & C, Mon 2:30 PM-4:00 PM 46th annual meeting, Orthopaedic Research Society. pp 151
Beynnon BD, Amis AA (1998) In vitro testing protocols for the cruciate ligaments and ligament reconstructions. Knee Surg Sports Traumatol Arthrosc 6:70–76. https://doi.org/10.1007/s001670050226
Beynnon BD, Johnson RJ, Toyama H et al (1994) The Relationship between anterior-posterior knee laxity and the structural properties of the patellar tendon graft: a study in canines. Am J Sports Med 22:812–820. https://doi.org/10.1177/036354659402200613
Bigliani LU, Pollock RG, Soslowsky LJ et al (1992) Tensile properties of the inferior glenohumeral ligament. J Orthop Res 10:187–197. https://doi.org/10.1002/jor.1100100205
Bonner TJ, Newell N, Karunaratne A et al (2015) Strain-rate sensitivity of the lateral collateral ligament of the knee. J Mech Behav Biomed Mater 41:261–270. https://doi.org/10.1016/j.jmbbm.2014.07.004
Burgio V, Civera M, Rodriguez Reinoso M et al (2022) Mechanical properties of animal tendons: a review and comparative study for the identification of the most suitable human tendon surrogates. Processes 10:485. https://doi.org/10.3390/pr10030485
Butler DL, Hulse DA, Kay MD et al (1983) Biomechanics of Cranial cruciate ligament reconstruction in the dog II mechanical properties. Vet Surg 12:113–118
Carballo CB, Hutchinson ID, Album ZM et al (2018) Biomechanics and microstructural analysis of the mouse knee and ligaments. J Knee Surg 31:520–527. https://doi.org/10.1055/s-0037-1604151
Chandrashekar N, Mansouri H, Slauterbeck J, Hashemi J (2006) Sex-based differences in the tensile properties of the human anterior cruciate ligament. J Biomech 39:2943–2950. https://doi.org/10.1016/j.jbiomech.2005.10.031
Ciccone WJ, Bratton DR, Weinstein DM et al (2006) Structural properties of lateral collateral ligament reconstruction at the fibular head. Am J Sports Med 34:24–28. https://doi.org/10.1177/0363546505278704
Comerford EJ, Tarlton JF, Innes JF et al (2005) Metabolism and composition of the canine anterior cruciate ligament relate to differences in knee joint mechanics and predisposition to ligament rupture. J Orthop Res 23:61–66. https://doi.org/10.1016/j.orthres.2004.05.016
Criscenti G, De Maria C, Sebastiani E et al (2016) Material and structural tensile properties of the human medial patello-femoral ligament. J Mech Behav Biomed Mater 54:141–148. https://doi.org/10.1016/j.jmbbm.2015.09.030
Dabbene I, Bullone M, Pagliara E et al (2018) Clinical findings and prognosis of interference injuries to the palmar aspect of the forelimbs in Standardbred racehorses: a study on 74 cases. Equine Vet J 50:759–765. https://doi.org/10.1111/evj.12836
Danto MI, Woo SL (1993) The mechanical properties of skeletally mature rabbit anterior cruciate ligament and patellar tendon over a range of strain rates. J Orthop Res 11:58–67. https://doi.org/10.1002/jor.1100110108
DeLong JM, Waterman BR (2015) Surgical Repair of Medial collateral ligament and posteromedial corner injuries of the knee: a systematic review. Arthrosc J Arthrosc Relat Surg 31:2249-2255.e5
Diotalevi L, Petit Y, Brailovski V et al (2018) Quasi-static tensile properties of the cranial cruciate ligament (CrCL) in adult cattle: towards the design of a prosthetic CrCL. J Mech Behav Biomed Mater 79:239–245. https://doi.org/10.1016/j.jmbbm.2017.12.024
Dupuis J, Harari J, Blackketter DM, Gallina AM (1994) Evaluation of the lateral collateral ligament after fibular head transposition in dogs. Vet Surg 23:456–465. https://doi.org/10.1111/j.1532-950X.1994.tb00507.x
Eleswarapu SV, Responte DJ, Athanasiou KA (2011) Tensile properties, collagen content, and crosslinks in connective tissues of the immature knee joint. PLoS ONE 6:1–7. https://doi.org/10.1371/journal.pone.0026178
El-Zawawy HB, Silva MJ, Sandell LJ, Wright RW (2005) Ligamentous versus physeal failure in murine medial collateral ligament biomechanical testing. J Biomech 38:703–706. https://doi.org/10.1016/j.jbiomech.2004.05.014
Figgie HE, Bahniuk EH, Heiple KG, Davy DT (1986) The effects of tibial-femoral angle on the failure mechanics of the canine anterior cruciate ligament. J Biomech 19:89–91. https://doi.org/10.1016/0021-9290(86)90139-9
Freedman BR, Baig HA, Guarino BB, Winkelstein BA (2012) Biomechanical effects of whole body vibration on spinal ligaments: a potential mechanism of tissue damage. In: 2012 38th annual northeast bioengineering conference, NEBEC, pp 398–399. https://doi.org/10.1109/NEBC.2012.6207132
Fremerey R, Bastian L, Siebert WE (2000) The coracoacromial ligament: anatomical and biomechanical properties with respect to age and rotator cuff disease. Knee Surg Sports Traumatol Arthrosc 8:309–313. https://doi.org/10.1007/s001670000135
Gellman KS, Bertram JEA (2002) The equine nuchal ligament 1: structural and material properties. Vet Comp Orthop Traumatol 15:01–06. https://doi.org/10.1055/s-0038-1632705
Germscheid NM, Thornton GM, Hart DA, Hildebrand KA (2011) A biomechanical assessment to evaluate breed differences in normal porcine medial collateral ligaments. J Biomech 44:725–731. https://doi.org/10.1016/j.jbiomech.2010.10.036
Gupte CM, Smith A, Jamieson N et al (2002) Meniscofemoral ligaments-structural and material properties. J Biomech 35:1623–1629. https://doi.org/10.1016/S0021-9290(02)00238-5
Gurlek AC, Sevinc B, Bayrak E, Erisken C (2017) Synthesis and characterization of polycaprolactone for anterior cruciate ligament regeneration. Mater Sci Eng C 71:820–826
Hewitt JD, Glisson RR, Guilak F, Vail TP (2002) The mechanical properties of the human hip capsule ligaments. J Arthroplast 17:82–89. https://doi.org/10.1054/arth.2002.27674
Hirokawa S, Sakoshita T (2003) An experimental study of the microstructures and mechanical properties of swine cruciate ligaments. JSME Int J Ser C Mech Syst, Mach Elem Manuf 46:1417–1425
Hunt P, Scheffler SU, Unterhauser FN, Weiler A (2005) A model of soft-tissue graft anterior cruciate ligament reconstruction in sheep. Arch Orthop Trauma Surg 125:238–248. https://doi.org/10.1007/s00402-004-0643-z
Jackson DW, Grood ES, Wilcox P et al (1988) The effects of processing techniques on the mechanical properties of bone-anterior cruciate ligament-bone allografts. An experimental study in goats. Am J Sports Med 16:101–105. https://doi.org/10.1177/036354658801600203
Jackson DW, Grood ES, Cohn BT et al (1991) The effects of in situ freezing on the anterior cruciate ligament. An experimental study in goats. J Bone Joint Surg Am 73:201–213
Jackson DW, Grood ES, Goldstein JD et al (1993) A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med 21:176–185. https://doi.org/10.1177/036354659302100203
Jansen MO, Savelberg HHCM (1994) Stress and strain of equine tendons of the forelimb at failure. Equine Vet J 26:57–60. https://doi.org/10.1111/j.2042-3306.1994.tb04875.x
Johnston JD, Small CF, Bouxsein ML, Pichora DR (2004) Mechanical properties of the scapholunate ligament correlate with bone mineral density measurements of the hand. J Orthop Res 22:867–871. https://doi.org/10.1016/j.orthres.2003.12.009
Kim KE, Hsu SL, Woo SLY (2014) Tensile properties of the medial patellofemoral ligament: the effect of specimen orientation. J Biomech 47:592–595. https://doi.org/10.1016/j.jbiomech.2013.11.026
Kusayama T, Harner CD, Carlin GJ et al (1994) Anatomical and biomechanical characteristics of human meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc 2:234–237. https://doi.org/10.1007/BF01845594
LaPrade RF, Bollom TS, Wentorf FA et al (2005) Mechanical properties of the posterolateral structures of the knee. Am J Sports Med 33:1386–1391. https://doi.org/10.1177/0363546504274143
Lee TQ, Dettling J, Sandusky MD, McMahon PJ (1999) Age related biomechanical properties of the glenoid-anterior band of the inferior glenohumeral ligament-humerus complex. Clin Biomech 14:471–476. https://doi.org/10.1016/S0268-0033(99)00007-8
Lee KE, Franklin AN, Davis MB, Winkelstein BA (2006) Tensile cervical facet capsule ligament mechanics: failure and subfailure responses in the rat. J Biomech 39:1256–1264. https://doi.org/10.1016/j.jbiomech.2005.03.018
Ma Y, Zhang X, Wang J et al (2009) Effect of bone morphogenetic protein-12 gene transfer on posterior cruciate ligament healing in a rabbit model. Am J Sports Med 37:599–609. https://doi.org/10.1177/0363546508325960
Mahalingam VD, Behbahani-Nejad N, Ronan EA et al (2015) Fresh versus frozen engineered bone-ligament-bone grafts for sheep anterior cruciate ligament repair. Tissue Eng Part C Methods 21:548–556. https://doi.org/10.1089/ten.tec.2014.0542
Mallett KF, Arruda EM (2017) Digital image correlation-aided mechanical characterization of the anteromedial and posterolateral bundles of the anterior cruciate ligament. Acta Biomater 56:44–57. https://doi.org/10.1016/j.actbio.2017.03.045
Martin RB, Burr DB, Sharkey NA, Fyhrie DP (2015) Mechanical properties of ligament and tendon. Skeletal tissue mechanics. Springer, New York, pp 175–225
Martins P, Silva-Filho AL, Fonseca AMRM et al (2013) Strength of round and uterosacral ligaments: a biomechanical study. Arch Gynecol Obstet 287:313–318. https://doi.org/10.1007/s00404-012-2564-3
Mattucci SFE, Moulton JA, Chandrashekar N, Cronin DS (2012) Strain rate dependent properties of younger human cervical spine ligaments. J Mech Behav Biomed Mater 10:216–226. https://doi.org/10.1016/j.jmbbm.2012.02.004
McPherson GK, Mendenhall HV, Gibbons DF et al (1985) Experimental mechanical and histologic evaluation of the Kennedy ligament augmentation device. Clin Orthop Relat Res 196:186–195. https://doi.org/10.1097/00003086-198506000-00025
Meller R, Willbold E, Hesse E et al (2008) Histologic and Biomechanical analysis of anterior cruciate ligament graft to bone healing in skeletally immature sheep. Arthrosc J Arthrosc Relat Surg 24:1221–1231. https://doi.org/10.1016/j.arthro.2008.06.021
Moon DK, Woo SLY, Takakura Y et al (2006) The effects of refreezing on the viscoelastic and tensile properties of ligaments. J Biomech 39:1153–1157. https://doi.org/10.1016/j.jbiomech.2005.02.012
Moore SM, McMahon PJ, Debski RE (2004) Bi-directional mechanical properties of the axillary pouch of the glenohumeral capsule: implications for modeling and surgical repair. J Biomech Eng 126:284–288. https://doi.org/10.1115/1.1695574
Moore SM, McMahon PJ, Azemi E, Debski RE (2005) Bi-directional mechanical properties of the posterior region of the glenohumeral capsule. J Biomech 38:1365–1369. https://doi.org/10.1016/j.jbiomech.2004.06.005
Murao T, Ochi M, Jitsuiki J, Ikuta Y (1997) The adverse effects of sectioning the posterior cruciate ligament in rabbits: changes in the structural and morphological properties of the femur-anterior cruciate ligament-tibia complex. Arch Orthop Trauma Surg 116:1–5. https://doi.org/10.1007/BF00434090
Nachemson AL, Evans JH (1968) Some mechanical properties of the third human lumbar interlaminar ligament (ligamentum flavum). J Biomech. https://doi.org/10.1016/0021-9290(68)90006-7
Nawata K, Enokida M, Yamasaki D et al (2001) Tensile properties of rat anterior cruciate ligament in collagen induced arthritis. Ann Rheum Dis 60:395–398. https://doi.org/10.1136/ard.60.4.395
Neumann P, Keller TS, Ekström L, Hansson T (1994) Effect of strain rate and bone mineral on the structural properties of the human anterior longitudinal ligament. Spine 19:205–211. https://doi.org/10.1097/00007632-199401001-00016
Ng GY, Oakes BW, Deacon OW et al (1995) Biomechanics of patellar tendon autograft for reconstruction of the anterior cruciate ligament in the goat: three-year study. J Orthop Res 13:602–608. https://doi.org/10.1002/jor.1100130416
Niehaus AJ, Anderson DE, Johnson JK, Lannutti JJ (2013) Comparison of the mechanical characteristics of polymerized caprolactam and monofilament nylon loops constructed in parallel strands or as braided ropes versus cranial cruciate ligaments of cattle. Am J Vet Res 74:381–385. https://doi.org/10.2460/ajvr.74.3.381
Nikolaou PK, Seaber AV, Glisson RR et al (1986) Anterior cruciate ligament allograft transplantation. Am J Sports Med 14:348–360. https://doi.org/10.1177/036354658601400502
Noyes F, Grood E (1976) The strength of the anterior cruciate ligament in humans and Rhesus monkeys. J Bone Joint Surg 58:1074–1082. https://doi.org/10.2106/00004623-197658080-00006
Noyes FR, DeLucas JL, Torvik PJ (1974) Biomechanics of anterior cruciate ligament failure: an analysis of strain rate sensitivity and mechanisms of failure in primates. J Bone Joint Surg Ser A 56:236–253. https://doi.org/10.2106/00004623-197456020-00002
Oskui IZ, Hashemi A, Jafarzadeh H (2016) Biomechanical behavior of bovine periodontal ligament: experimental tests and constitutive model. J Mech Behav Biomed Mater 62:599–606. https://doi.org/10.1016/j.jmbbm.2016.05.036
Panjabi MM, Yoldas E, Oxland TR, Crisco JJ (1996) Subfailure injury of the rabbit anterior cruciate ligament. J Orthop Res 14:216–222. https://doi.org/10.1002/jor.1100140208
Pieroh P, Schneider S, Lingslebe U et al (2016) The stress-strain data of the hip capsule ligaments are gender and side independent suggesting a smaller contribution to passive stiffness. PLoS ONE 11:1–16. https://doi.org/10.1371/journal.pone.0163306
Pioletti DP, Rakotomanana LR, Leyvraz PF (1999) Strain rate effect on the mechanical behavior of the anterior cruciate ligament-bone complex. Med Eng Phys 21(2):95–100. https://doi.org/10.1016/s1350-4533(99)00028-4
Polak K, Czyz M, Ścigała K et al (2014) Biomechanical characteristics of the porcine denticulate ligament in different vertebral levels of the cervical spine-Preliminary results of an experimental study. J Mech Behav Biomed Mater 34:165–170. https://doi.org/10.1016/j.jmbbm.2014.02.010
Przybylski GJ, Carlin GJ, Patel PR, Woo SLY (1996) Human anterior and posterior cervical longitudinal ligaments possess similar tensile properties. J Orthop Res 14:1005–1008. https://doi.org/10.1002/jor.1100140623
Quapp KM, Weiss JA (1997) A material characterization of human medial collateral ligament. In: American society of mechanical engineers, bioengineering division (Publication) BED, vol 36, pp 191–192. https://doi.org/10.1115/imece1997-0293
Quinn KP, Winkelstein BA (2007) Cervical facet capsular ligament yield defines the threshold for injury and persistent joint-mediated neck pain. J Biomech 40:2299–2306. https://doi.org/10.1016/j.jbiomech.2006.10.015
Race A, Amis AA (1994) The mechanical properties of the two bundles of the human posterior cruciate ligament. J Biomech 27:13–24. https://doi.org/10.1016/0021-9290(94)90028-0
Radford WJP, Amis AA, Stead AC (1996) The ovine stifle as a model for human cruciate ligament surgery. Vet Comp Orthop Traumatol 09:134–139. https://doi.org/10.1055/s-0038-1632518
Riemersma DJ, Schamhardt HC (1985) In vitro mechanical properties of equine tendons in relation to cross-sectional area and collagen content. Res Vet Sci 39:263–270. https://doi.org/10.1016/S0034-5288(18)31711-9
Robinson JR, Bull AMJ, Amis AA (2005) Structural properties of the medial collateral ligament complex of the human knee. J Biomech 38:1067–1074. https://doi.org/10.1016/j.jbiomech.2004.05.034
Rogers GJ, Milthorpe BK, Muratore A, Schindhelm K (1990) Measurement of the mechanical properties of the ovine anterior cruciate ligament bone-ligament-bone complex: a basis for prosthetic evaluation. Biomaterials 11:89–96. https://doi.org/10.1016/0142-9612(90)90122-7
Rumian AP, Wallace AL, Birch HL (2007) Tendons and ligaments are anatomically distinct but overlap in molecular and morphological features—a comparative study in an ovine model. J Orthop Res 25:458–464. https://doi.org/10.1002/jor.20218
Sample SJ (2017) Biomechanics of the cruciate ligaments. Advances in the canine cranial cruciate ligament, 2nd edn. Wiley, Hoboken, pp 13–20. https://doi.org/10.1002/9781119261728.ch2
Schleifenbaum S, Prietzel T, Hädrich C et al (2016) Tensile properties of the hip joint ligaments are largely variable and age-dependent–an in-vitro analysis in an age range of 14–93 years. J Biomech 49:3437–3443. https://doi.org/10.1016/j.jbiomech.2016.09.001
Shetye SS, Malhotra K, Ryan SD, Puttlitz CM (2009) Determination of mechanical properties of canine carpal ligaments. Am J Vet Res 70:1026–1030. https://doi.org/10.2460/ajvr.70.8.1026
Shino K, Kawasaki T, Hirose H et al (1984) Replacement of the anterior cruciate ligament by an allogeneic tendon graft. An experimental study in the dog. J Bone Joint Surg Br 66-B:672–681. https://doi.org/10.1302/0301-620X.66B5.6501359
Smith TJ (2006) The Relationship between tendon morphology and function, PhD Thesis. University College London
Su WR, Chen HH, Luo ZP (2008) Effect of cyclic stretching on the tensile properties of patellar tendon and medial collateral ligament in rat. Clin Biomech 23:911–917. https://doi.org/10.1016/j.clinbiomech.2008.04.002
Sugita T, Amis AA (2001) Anatomic and biomechanical study of the lateral collateral and popliteofibular ligaments. Am J Sports Med 29:466–472. https://doi.org/10.1177/03635465010290041501
Tan T, Davis FM, Gruber DD et al (2015) Histo-mechanical properties of the swine cardinal and uterosacral ligaments. J Mech Behav Biomed Mater 42:129–137. https://doi.org/10.1016/j.jmbbm.2014.11.018
Tozilli PA, Arnoczky SP (1988) Mechanical properties of the lateral collateral ligament: effect of cruciate instability in the rabbit. J Biomech Eng 110:208–212. https://doi.org/10.1115/1.3108432
Trent PS, Walker PS, Wolf B (1976) Ligament length patterns, strength, and rotational axes of the knee joint. Clin Orthop Relat Res 117:263–270
Vardy MD, Gardner TR, Cosman F et al (2005) The effects of hormone replacement on the biomechanical properties of the uterosacral and round ligaments in the monkey model. Am J Obstet Gynecol 192:1741–1751. https://doi.org/10.1016/j.ajog.2004.10.639
Viateau V, Manassero M, Anagnostou F et al (2013) Biological and biomechanical evaluation of the ligament advanced reinforcement system (LARS AC) in a sheep model of anterior cruciate ligament replacement: a 3-month and 12-month study. Arthrosc J Arthrosc Relat Surg 29:1079–1088. https://doi.org/10.1016/j.arthro.2013.02.025
Weiler A, Peters G, Mäurer J et al (2001) Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging: a two-year study in sheep. Am J Sports Med 29:751–761. https://doi.org/10.1177/03635465010290061401
Weiler A, Förster C, Hunt P et al (2004) The influence of locally applied platelet-derived growth factor-BB on free tendon graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med 32:881–891. https://doi.org/10.1177/0363546503261711
Weiss JA, Woo SL, Ohland KJ et al (1991) Evaluation of a new injury model to study medial collateral ligament healing: primary repair versus nonoperative treatment. J Orthop Res 9:516–528. https://doi.org/10.1002/jor.1100090407
Wijdicks CA, Ewart DT, Nuckley DJ et al (2010) Structural properties of the primary medial knee ligaments. Am J Sports Med 38:1638–1646. https://doi.org/10.1177/0363546510363465
Wilson WT, Deakin AH, Payne AP et al (2012) Comparative analysis of the structural properties of the collateral ligaments of the human knee. J Orthop Sports Phys Ther 42:345–351. https://doi.org/10.2519/jospt.2012.3919
Wingfield C, Amis AA, Stead AC, Law HT (2000) Comparison of the biornechanical properties of rottweiler and racing greyhound cranial cruciate ligaments. J Small Anim Pract 41:303–307. https://doi.org/10.1111/j.1748-5827.2000.tb03206.x
Woo SL-Y, Orlando CA, Camp JF, Akeson WH (1986) Effects of postmortem storage by freezing on ligament tensile behavior. J Biomech 19:399–404. https://doi.org/10.1016/0021-9290(86)90016-3
Woo SL, Peterson RH, Ohland KJ et al (1990a) The effects of strain rate on the properties of the medial collateral ligament in skeletally immature and mature rabbits: a biomechanical and histological study. J Orthop Res 8:712–721. https://doi.org/10.1002/jor.1100080513
Woo SL, Young EP, Ohland KJ et al (1990b) The effects of transection of the anterior cruciate ligament on healing of the medial collateral ligament. A biomechanical study of the knee in dogs. J Bone Joint Surg Am 72:382–392
Woo SL-Y, Ohland KJ, Weiss JA (1990c) Aging and sex-related changes in the biomechanical properties of the rabbit medial collateral ligament. Mech Ageing Dev 56:129–142. https://doi.org/10.1016/0047-6374(90)90004-Y
Woo SL-Y, Hollis JM, Adams DJ et al (1991) Tensile properties of the human femur-anterior cruciate ligament-tibia complex. Am J Sports Med 19:217–225. https://doi.org/10.1177/036354659101900303
Woo SLY, Newton PO, MacKenna DA, Lyon RM (1992) A comparative evaluation of the mechanical properties of the rabbit medial collateral and anterior cruciate ligaments. J Biomech 25:377–386. https://doi.org/10.1016/0021-9290(92)90257-2
Xie WQ, He M, He YQ et al (2021) The effects of posterior cruciate ligament rupture on the biomechanical and histological characteristics of the medial collateral ligament: an animal study. J Orthop Surg Res 16:1–9. https://doi.org/10.1186/s13018-021-02443-0
Yiannakopoulos CK, Kanellopoulos AD, Dontas IA et al (2005) The symmetry of the medial collateral and anterior cruciate ligament properties. A biomechanical study in the rat hind limb. J Musculoskelet Neuronal Interact 5:170–173
Zens M, Feucht MJ, Ruhhammer J et al (2015) Mechanical tensile properties of the anterolateral ligament. J Exp Orthop 2:7. https://doi.org/10.1186/s40634-015-0023-3
Zhou T, Grimshaw PN, Jones C (2009) A biomechanical investigation of the anteromedial and posterolateral bands of the porcine anterior cruciate ligament. Proc Inst Mech Eng Part H: J Eng Med 223(6):767–775. https://doi.org/10.1243/09544119JEIM483
Funding
Open access funding provided by Politecnico di Torino within the CRUI-CARE Agreement. This work was supported by EUREKA! venture SGR spa under the framework of the project T-REM3DIE (Tendon REpair MEdical DevIcE).
Author information
Authors and Affiliations
Contributions
Conceptualisation was performed by VB, MC, MRR, AB, and CS; methodology by VB and MC; validation, VB, MC, MRR, SC., MM, FS, GS; formal analysis by VB, MC, MRR, SC, MM, FS, GS; investigation by VB, MC, MRR, SC, MM, FS, GS; resources, AB and CS; data curation by VB, MC, MRR, SC, MM, FS, GS; writing—original draft preparation by VB, SC, MM, FS, GS; writing—review and editing by MC, MRR, AB, and CS; visualisation by VB, SC, MM, FS, GS; supervision by MC, MRR, AB, and CS; project administration by MC, MRR, AB, and CS; funding acquisition by CS. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burgio, V., Casari, S., Milizia, M. et al. Mechanical properties of animal ligaments: a review and comparative study for the identification of the most suitable human ligament surrogates. Biomech Model Mechanobiol 22, 1645–1683 (2023). https://doi.org/10.1007/s10237-023-01718-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-023-01718-1