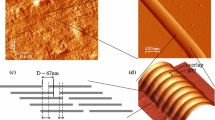
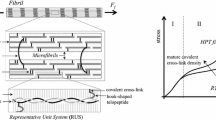
Abstract
Collagen is an abundant structural biopolymer in mammal vertebrates, providing structural support as well as mechanical integrity for connective tissues such as bone, ligament, and tendon. The mechanical behaviours of these tissues are determined by the nanomechanics of their structures at different hierarchies and the role of collagen structures in the extracellular matrix. Some studies revealed that there is significant microstructural difference in the longitudinal direction of the collagen fibril, which challenges the conventional rod-like assumption prevalently adopted in the existing studies. Motivated by this discrepancy, in this study, we investigated the longitudinal heterogeneous nanomechanical properties of type I collagen molecule to probe the origin of the longitudinal heterogeneity of the collagen fibril at the molecular level. A full length type I collagen molecule structure was built based on the experimentally calibrated nanostructure. Then, a suitable strain rate was determined for stretching the three intact ‘gap’ regions and three intact ‘overlap’ regions of the collagen molecule. Further, the nanomechanical properties of the six collagen molecule segments were characterized by performing steered molecular dynamics simulations, using the obtained suitable strain rate in modelling. The results indicate that this computational model can be used to capture the mechanical behaviour of the collagen molecule under physiological stress conditions. Moreover, the ‘gap’ regions show a lower stiffness and undergo a slightly lager strain in the unwinding process, compared to the ‘overlap’ regions of the collagen molecule. This investigation provides insights into the origin of the longitudinal heterogeneity of collagen fibrils at the molecular level and suggests that it is of significant importance to consider the longitudinal heterogeneous mechanical properties of the collagen molecule in the development of coarse-grained models of collagen-related tissues.







Similar content being viewed by others
References
Baldwin SJ, Quigley AS, Clegg C, Kreplak L (2014) Nanomechanical mapping of hydrated rat tail tendon collagen I fibrils. Biophys J 107:1794–1801
Balooch M, Habelitz S, Kinney J, Marshall S, Marshall G (2008) Mechanical properties of mineralized collagen fibrils as influenced by demineralization. J Struct Biol 162:404–410
Berendsen HJ, Postma JP, van Gunsteren WF, Hermans J (1981) Interaction models for water in relation to protein hydration. In: Pullman B (ed) Intermolecular forces. Springer, Berlin, pp 331–342
Berendsen HJ, van der Spoel D, van Drunen R (1995) GROMACS: a message-passing parallel molecular dynamics implementation. Comput Phys Commun 91:43–56
Bodian DL, Radmer RJ, Holbert S, Klein TE (2011) Molecular dynamics simulations of the full triple helical region of collagen type I provide an atomic scale view of the protein’s regional heterogeneity. In: Pacific symposium on biocomputing. pacific symposium on biocomputing, p. 193
Bozec L, Horton M (2005) Topography and mechanical properties of single molecules of type I collagen using atomic force microscopy. Biophys J 88:4223–4231
Buehler MJ (2006a) Atomistic and continuum modeling of mechanical properties of collagen: elasticity, fracture, and self-assembly. J Mater Res 21:1947–1961
Buehler MJ (2006b) Nature designs tough collagen: explaining the nanostructure of collagen fibrils. Proc Natl Acad Sci 103:12285–12290
Buehler MJ (2008) Nanomechanics of collagen fibrils under varying cross-link densities: atomistic and continuum studies. J Mech Behav Biomed Mater 1:59–67
Cusack S, Miller A (1979) Determination of the elastic constants of collagen by Brillouin light scattering. J Mol Biol 135:39–51
Depalle B, Qin Z, Shefelbine SJ, Buehler MJ (2014) Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J Mech Behav Biomed Mater 52:1–13
Eppell S, Smith B, Kahn H, Ballarini R (2006) Nano measurements with micro-devices: mechanical properties of hydrated collagen fibrils. J R Soc Interface 3:117–121
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103:8577–8593
Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H, Bernstorff S (1998) Fibrillar structure and mechanical properties of collagen. J Struct Biol 122:119–122
Gautieri A, Vesentini S, Montevecchi FM, Redaelli A (2008) Mechanical properties of physiological and pathological models of collagen peptides investigated via steered molecular dynamics simulations. J Biomech 41:3073–3077
Gautieri A, Buehler MJ, Redaelli A (2009a) Deformation rate controls elasticity and unfolding pathway of single tropocollagen molecules. J Mech Behav Biomed Mater 2:130–137
Gautieri A, Vesentini S, Redaelli A, Buehler MJ (2009b) Single molecule effects of osteogenesis imperfecta mutations in tropocollagen protein domains. Protein Sci 18:161–168
Gautieri A, Russo A, Vesentini S, Redaelli A, Buehler MJ (2010) Coarse-grained model of collagen molecules using an extended MARTINI force field. J Chem Theory Comput 6:1210–1218
Gautieri A, Vesentini S, Redaelli A, Buehler MJ (2011) Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Lett 11:757–766
Gautieri A, Vesentini S, Redaelli A, Buehler MJ (2012a) Viscoelastic properties of model segments of collagen molecules. Matrix Biol 31:141–149
Gautieri A, Vesentini S, Redaelli A, Buehler MJ (2012b) Osteogenesis imperfecta mutations lead to local tropocollagen unfolding and disruption of H-bond network. RSC Adv 2:3890–3896
Gautieri A, Vesentini S, Redaelli A, Ballarini R (2013) Modeling and measuring visco-elastic properties: from collagen molecules to collagen fibrils. Int J Non-Linear Mech 56:25–33
Gentleman E, Lay AN, Dickerson DA, Nauman EA, Livesay GA, Dee KC (2003) Mechanical characterization of collagen fibers and scaffolds for tissue engineering. Biomaterials 24:3805–3813
Gopalakrishnan R, Singam EA, Sundar JV, Subramanian V (2015) Interaction of collagen like peptides with gold nanosurfaces: a molecular dynamics investigation. Phys Chem Chem Phys 17:5172–5186
Graham JS, Vomund AN, Phillips CL, Grandbois M (2004) Structural changes in human type I collagen fibrils investigated by force spectroscopy. Exp Cell Res 299:335–342
Gupta H, Messmer P, Roschger P, Bernstorff S, Klaushofer K, Fratzl P (2004) Synchrotron diffraction study of deformation mechanisms in mineralized tendon. Phys Rev Lett 93:158101
Harley R, James D, Miller A, White J (1977) Phonons and the elastic moduli of collagen and muscle. Nat 267:285–287. doi:10.1038/267285a0
Heim AJ, Matthews WG, Koob TJ (2006) Determination of the elastic modulus of native collagen fibrils via radial indentation. Appl Phys Lett 89:181902
Hess B, Bekker H, Berendsen HJ, Fraaije JG (1997) LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18:1463–1472
Hodge AJ, Schmitt FO (1960) The charge profile of the tropocollagen macromolecule and the packing arrangement in native-type collagen fibrils. Proc Natl Acad Sci USA 46:186
Hofmann H, Voss T, Kühn K, Engel J (1984) Localization of flexible sites in thread-like molecules from electron micrographs: comparison of interstitial, basement membrane and intima collagens. J Mol Biol 172:325–343
Hulmes D (2008) Collagen diversity, synthesis and assembly. In: Fratzl P (ed) Collagen. Springer, Berlin, pp 15–47
Husar-Memmer E, Ekici A, Al Kaissi A, Sticht H, Manger B, Schett G et al (2013) Premature osteoarthritis as presenting sign of type II collagenopathy: a case report and literature review. In: Seminars in arthritis and rheumatism, pp 355–360
Jenkins CL, Raines RT (2002) Insights on the conformational stability of collagen. Nat Prod Rep 19:49–59
Kadler KE, Baldock C, Bella J, Boot-Handford RP (2007) Collagens at a glance. J Cell Sci 120:1955–1958
Kim J-H, Lee G, Won Y, Lee M, Kwak J-S, Chun C-H et al (2015) Matrix cross-linking-mediated mechanotransduction promotes posttraumatic osteoarthritis. Proc Natl Acad Sci 112:9424–9429
Leistritz DF, Pepin MG, Schwarze U, Byers PH (2011) COL3A1 haploinsufficiency results in a variety of Ehlers–Danlos syndrome type IV with delayed onset of complications and longer life expectancy. Genet Med 13:717–722
Lindahl K, Åström E, Rubin C-J, Grigelioniene G, Malmgren B, Ljunggren Ö et al (2015) Genetic epidemiology, prevalence, and genotype-phenotype correlations in the Swedish population with osteogenesis imperfecta. Eur J Hum Genet 23:1042–1050
Lorenzo AC, Caffarena ER (2005) Elastic properties, Young’s modulus determination and structural stability of the tropocollagen molecule: a computational study by steered molecular dynamics. J Biomech 38:1527–1533
Maupetit J, Gautier R, Tufféry P (2006) SABBAC: online Structural Alphabet-based protein BackBone reconstruction from Alpha-Carbon trace. Nucleic Acids Res 34:W147–W151
Minary-Jolandan M, Yu M-F (2009) Nanomechanical heterogeneity in the gap and overlap regions of type I collagen fibrils with implications for bone heterogeneity. Biomacromolecules 10:2565–2570
Orgel JP, Irving TC, Miller A, Wess TJ (2006) Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci 103:9001–9005
Orgel JP, Persikov AV, Antipova O (2014) Variation in the helical structure of native collagen. PloS ONE 9:e89519
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182–7190
Pradhan SM, Katti DR, Katti KS (2011) Steered molecular dynamics study of mechanical response of full length and short collagen molecules. J Nanomech Micromech 1:104–110
Ravikumar K, Humphrey J, Hwang W (2007) Spontaneous unwinding of a labile domain in a collagen triple helix. J Mech Mater Struct 2:999–1010
Robin NH, Moran RT, Ala-Kokko L (2014) Stickler syndrome In: Pagon RA, Adam MP, Ardinger HH et al (eds) GeneReviews(r) [Internet], 1993–2016. University of Washington, Seattle. http://www.ncbi.nlm.nih.gov/books/NBK1302/
Robinson R, Watson M (1952) Collagen–crystal relationships in bone as seen in the electron microscope. Anat Rec 114:383–409
Sasaki N, Odajima S (1996) Elongation mechanism of collagen fibrils and force–strain relations of tendon at each level of structural hierarchy. J Biomech 29:1131–1136
Shen ZL, Dodge MR, Kahn H, Ballarini R, Eppell SJ (2008) Stress–strain experiments on individual collagen fibrils. Biophys J 95:3956–3963
Sherman VR, Yang W, Meyers MA (2015) The materials science of collagen. J Mech Behav Biomed Mater 52:22–50
Spitzner E-C, Röper S, Zerson M, Bernstein A, Magerle R (2015) Nanoscale swelling heterogeneities in type I collagen fibrils. ACS Nano 9:5683–5694
Stevens MJ (2008) Simulation of the mechanical strength of a single collagen molecule. Biophys J 95:33–39
Streeter I, de Leeuw NH (2011) A molecular dynamics study of the interprotein interactions in collagen fibrils. Soft Matter 7:3373–3382
Sun Y-L, Luo Z-P, Fertala A, An K-N (2002) Direct quantification of the flexibility of type I collagen monomer. Biochem Biophys Res Commun 295:382–386
Sun Y-L, Luo Z-P, Fertala A, An K-N (2004) Stretching type II collagen with optical tweezers. J Biomech 37:1665–1669
Uzel SG, Buehler MJ (2009) Nanomechanical sequencing of collagen: tropocollagen features heterogeneous elastic properties at the nanoscale. Integr Biol 1:452–459
van der Rijt JA, van der Werf KO, Bennink ML, Dijkstra PJ, Feijen J (2006) Micromechanical testing of individual collagen fibrils. Macromol Biosci 6:697–702
Vesentini S, Fitié CF, Montevecchi FM, Redaelli A (2005) Molecular assessment of the elastic properties of collagen-like homotrimer sequences. Biomech Model Mechanobiol 3:224–234
Vesentini S, Redaelli A, Gautieri A (2013) Nanomechanics of collagen microfibrils. Muscles Ligaments Tendons J 3:23
Weiner S, Wagner HD (1998) The material bone: structure-mechanical function relations. Annu Rev Mater Sci 28:271–298
Wenger MP, Bozec L, Horton MA, Mesquida P (2007) Mechanical properties of collagen fibrils. Biophys J 93:1255–1263
Wenger MP, Mesquida P (2011) Longitudinal variations in the Poisson’s ratio of collagen fibrils. Appl Phys Lett 98:163707
Yang W, Sherman VR, Gludovatz B, Schaible E, Stewart P, Ritchie RO et al (2015) On the tear resistance of skin. Nat Commun 6
Zhou Z, Minary-Jolandan M, Qian D (2015) A simulation study on the significant nanomechanical heterogeneous properties of collagen. Biomech Model Mechanobiol 14(3): 445–457
Acknowledgements
The authors thank Joseph Orgel (Center For Molecular Study Of Condensed Soft Matter, Pritzker Institute of Biomedical Science and Engineering at the Illinois Institute of Technology and Argonne National Lab, USA) for his help on dividing the gap and overlap regions of the collagen molecule. This research was funded by ARC Discovery Project (DP150100828). The High Performance Computer resources provided by Queensland University of Technology (QUT) are gratefully acknowledged. Also the financial support of China Scholarship Council (CSC) scholarship from Chinese government and Top-up scholarship from QUT are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tang, M., Li, T., Gandhi, N.S. et al. Heterogeneous nanomechanical properties of type I collagen in longitudinal direction. Biomech Model Mechanobiol 16, 1023–1033 (2017). https://doi.org/10.1007/s10237-016-0870-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-016-0870-6