Abstract
Blebs are cellular protrusions that are used by cells for multiple purposes including locomotion. A mechanical model for the problem of pressure-driven blebs based on force and moment balances of an axisymmetric shell model is proposed. The formation of a bleb is initiated by weakening the shell over a small region, and the deformation of the cellular membrane from the cortex is obtained during inflation. However, simply weakening the shell leads to an area increase of more than 4 %, which is physically unrealistic. Thus, the model is extended to include a reconfiguration process that allows large blebs to form with small increases in area. It is observed that both geometric and biomechanical constraints are important in this process. In particular, it is shown that although blebs are driven by a pressure difference across the cellular membrane, it is not the limiting factor in determining bleb size.
















Similar content being viewed by others
References
Ashby MF, Gibson LJ, Wegst U, Olive R (1995) The mechanical properties of natural materials. I. Material property charts. Proc R Soc A 450(1938):123–140
Blaser H, Reichman-Fried M, Castanon I, Dumstrei K, Marlow FL, Kawakami K, Solnica-Krezel L, Heisenberg CP, Raz E (2006) Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev Cell 11(5):613–627
Boucrot E, Kirchhausen T (2007) Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci 104(19):7939
Charras GT (2008) A short history of blebbing. J Microsc 231(3):466–478
Charras G, Paluch E (2008) Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol 9(9):730–736
Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ (2005) Non-equilibration of hydrostatic pressure in blebbing cells. Nature 435(7040):365–369
Charras GT, Coughlin M, Mitchison TJ, Mahadevan L (2008) Life and times of a cellular bleb. Biophys J 94(5):1836–1853
Collins-Hooper H, Woolley TE, Dyson L, Patel A, Potter P, Baker RE, Gaffney EA, Maini PK, Dash PR, Patel K (2012) Age-related changes in speed and mechanism of adult skeletal muscle stem cell migration. Stem Cells 30(6):1182–1195
Cunningham CC (1995) Actin polymerization and intracellular solvent flow in cell surface blebbing. J Cell Biol 129(6):1589–1599
Dai J, Sheetz MP (1999) Membrane tether formation from blebbing cells. Biophys J 77(6):3363–3370
Evans EA (1983) Bending elastic modulus of red blood cell membrane derived from buckling instability in micropipet aspiration tests. Biophys J 43(1):27–30
Evans EA, Skalak R (1980) Mechanics and thermodynamics of biomembranes. CRC Press, Boca Raton, FL
Fackler OT, Grosse R (2008) Cell motility through plasma membrane blebbing. J Cell Biol 181(6):879
Goriely A, Tabor M (2003a) Biomechanical models of hyphal growth in actinomycetes. J Theor Biol 222(2):211–218
Goriely A, Tabor M (2003b) Self-similar tip growth in filamentary organisms. Phys Rev Lett 90(10):108101
Goriely A, Tabor M (2006) Estimates of biomechanical forces in Magnaporthe grisea. Mycol Res 110(Pt 7):755–759
Goriely A, Károlyi G, Tabor M (2005) Growth induced curve dynamics for filamentary micro-organisms. J Math Biol 51(3):355–366
Hallett MB, Dewitt S (2007) Ironing out the wrinkles of neutrophil phagocytosis. Trends Cell Biol 17(5):209–214
Hallett MB, von Ruhland CJ, Dewitt S (2008) Chemotaxis and the cell surface-area problem. Nat Rev Mol Cell Biol 9(8):662–662
Hickson GRX, Echard A, O’Farrell PH (2006) Rho-kinase controls cell shape changes during cytokinesis. Curr Biol 16(4):359–370
Hu J (2009) Mathematical modeling and analysis of in vitro Actin filament dynamics and cell blebbing. PhD thesis, University of Minnesota
Keller H, Eggli P (1998) Protrusive activity, cytoplasmic compartmentalization, and restriction rings in locomoting blebbing Walker carcinosarcoma cells are related to detachment of cortical actin from the plasma membrane. Cell Motil Cytoskelet 41(2):181–193
Keller HU, Bebie H (1996) Protrusive activity quantitatively determines the rate and direction of cell locomotion. Cell Motil Cytoskelet 33(4):241–251
Lew DJ (2003) The morphogenesis checkpoint: how yeast cells watch their figures. Curr Opin Cell Biol 15(6):648–653
Lim FY, Chiam KH, Mahadevan L (2012) The size, shape, and dynamics of cellular blebs. Bull Am Phys Soc 55:1–6
Maugis B, Brugués J, Nassoy P, Guillen N, Sens P, Amblard F (2010) Dynamic instability of the intracellular pressure drives bleb-based motility. J Cell Sci 123(22):3884–3892
Mills JC, Stone NL, Erhardt J, Pittman RN (1998) Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol 140(3):627–636
Nichol JA, Hutter OF (1996) Tensile strength and dilatational elasticity of giant sarcolemmal vesicles shed from rabbit muscle. J Phys 493:187
Norman LL, Brugs J, Sengupta K, Sens P, Aranda-Espinoza H (2010) Cell blebbing and membrane area homeostasis in spreading and retracting cells. Biophys J 99(6):1726–1733
Otto A, Collins-Hooper H, Patel A, Dash PR, Patel K (2011) Adult skeletal muscle stem cell migration is mediated by a blebbing/amoeboid mechanism. Rejuv Res 14(3):249–260
Paluch E, Sykes C, Prost J, Bornens M (2006) Dynamic modes of the cortical actomyosin gel during cell locomotion and division. Trends Cell Biol 16(1):5–10
Pozrikidis C (2001) Effect of membrane bending stiffness on the deformation of capsules in simple shear flow. J Fluid Mech 440:269–291
Pressley A (2010) Elementary differential geometry. Springer, New York
Robertson AMG, Bird CC, Waddell AW, Currie AR (1978) Morphological aspects of glucocorticoid-induced cell death in human lymphoblastoid cells. J Pathol 126(3):181–187
Russell SW, Rosenau W, Lee JC (1972) Cytolysis induced by human lymphotoxin: cinemicrographic and electron microscopic observations. Am J Pathol 69(1):103
Sahai E, Marshall CJ (2003) Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 5(8):711–719
Schütz K, Keller H (1998) Protrusion, contraction and segregation of membrane components associated with passive deformation and shape recovery of Walker carcinosarcoma cells. Eur J Cell Biol 77(2):100–110
Sheetz MP, Sable JE, Döbereiner HG (2006) Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu Rev Biophys Biomol Struct 35:417–434
Skalak R, Tozeren A, Zarda RP, Chien S (1973) Strain energy function of red blood cell membranes. Biophys J 13(3):245–264
Spangler EJ, Harvey CW, Revalee JD, Sunil Kumar PB, Laradji M (2011) Computer simulation of cytoskeleton-induced blebbing in lipid membranes. Phys Rev E 84:051906
Strychalski W, Guy RD (2012) A computational model of bleb formation. Math Med Biol 30:115–130
Tinevez JY, Schulze U, Salbreux G, Roensch J, Joanny JF, Paluch E (2009) Role of cortical tension in bleb growth. Proc Natl Acad Sci 106(44):18581–18586
Tongen A, Goriely A, Tabor M (2006) Biomechanical model for appressorial design in Magnaporthe grisea. J Theor Biol 240(1):1–8
Young J, Mitran S (2010) A numerical model of cellular blebbing: a volume-conserving, fluid-structure interaction model of the entire cell. J Biomech 43:210–220
Acknowledgments
This publication is based on work supported by Award No. KUK-C1-013-04, made by King Abdullah University of Science and Technology (KAUST). AG is a Wolfson Royal Society Merit Holder and acknowledges support from a Reintegration Grant under EC Framework VII.
Author information
Authors and Affiliations
Corresponding author
Appendix: Geometric model
Appendix: Geometric model
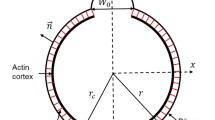
In this appendix, we summarise the equations that relate the radius, R, of an initial sphere to the radii, \(r_b\) and \(r_c\), of an equivalent volume system of two spheres connected through a pinned neck region, see Fig. 17. We follow the geometric construction of a blebbing cell as derived by Hu (2009).
From Fig. 17, we see that the equality
must always hold. Further, we demand that the cell volume be constant,
Finally, we define the bleb volume fraction, x, as \(v_b=xV\), and hence, the cell volume fraction is \(v_c=(1-x)V\). This parameter \(x\) is an input to the problem.
From Eqs. (39) and (40), we can derive equations satisfied by the radii and connection angles:
where \(R,\,\varTheta \) and x all input parameters. Observe that x is varied from the initial bleb volume ratio,
to the maximal bleb ratio, \(1-x_0\).
Originally, R was taken as a free variable (Hu 2009). However, using Eqs. (19)–(25) and assuming spherical symmetry of the solution, we can derive the initial radius of the unweakened cell, R. The unique solution can be seen to be:
Substituting these back into the constitutive Eq. (16), we find
The positive root of this quadratic, which depends on \(\varDelta P,\,\rho ,\,A\) and \(\mu \), then provides the desired radius.
Rights and permissions
About this article
Cite this article
Woolley, T.E., Gaffney, E.A., Oliver, J.M. et al. Cellular blebs: pressure-driven, axisymmetric, membrane protrusions. Biomech Model Mechanobiol 13, 463–476 (2014). https://doi.org/10.1007/s10237-013-0509-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-013-0509-9