Abstract
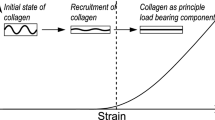
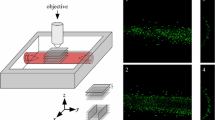
Mechanical properties of the adventitia are largely determined by the organization of collagen fibers. Measurements on the waviness and orientation of collagen, particularly at the zero-stress state, are necessary to relate the structural organization of collagen to the mechanical response of the adventitia. Using the fluorescence collagen marker CNA38-OG488 and confocal laser scanning microscopy, we imaged collagen fibers in the adventitia of rabbit common carotid arteries ex vivo. The arteries were cut open along their longitudinal axes to get the zero-stress state. We used semi-manual and automatic techniques to measure parameters related to the waviness and orientation of fibers. Our results showed that the straightness parameter (defined as the ratio between the distances of endpoints of a fiber to its length) was distributed with a beta distribution (mean value 0.72, variance 0.028) and did not depend on the mean angle orientation of fibers. Local angular density distributions revealed four axially symmetric families of fibers with mean directions of 0°, 90°, 43° and −43°, with respect to the axial direction of the artery, and corresponding circular standard deviations of 40°, 47°, 37° and 37°. The distribution of local orientations was shifted to the circumferential direction when measured in arteries at the zero-load state (intact), as compared to arteries at the zero-stress state (cut-open). Information on collagen fiber waviness and orientation, such as obtained in this study, could be used to develop structural models of the adventitia, providing better means for analyzing and understanding the mechanical properties of vascular wall.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Axer H, Keyserlingk DGV, Prescher A (2001) Collagen fibers in linea alba and rectus sheaths: II. Variability and biomechanical aspects. J Surg Res 96(2): 239–245
Bayan C, Levitt JM, Miller E, Kaplan D, Georgakoudi I (2009) Fully automated, quantitative, noninvasive assessment of collagen fiber content and organization in thick collagen gels. J Appl Phys 105(10): 1–11
Bigun J, Bigun T, Nilsson K (2004) Recognition by symmetry derivatives and the generalized structure tensor. IEEE Trans Pattern Anal Mach Intell 26(12): 1590–1605
Billiar KL, Sacks MS (1997) A method to quantify the fiber kinematics of planar tissues under biaxial stretch. J Biomech 30(7): 753–756
Boerboom RA, Krahn KN, Megens RT, van Zandvoort MA, Merkx M, Bouten CV (2007) High resolution imaging of collagen organisation and synthesis using a versatile collagen specific probe. J Struct Biol 159(3): 392–399
Boulesteix T, Pena AM, Pagès N, Godeau G, Sauviat MP, Beaurepaire E, Schanne-Klein MC (2006) Micrometer scale ex vivo multiphoton imaging of unstained arterial wall structure. Cytometry Part A 69(1): 20–26
Braga-Vilela AS, Pimentel ER, Marangoni S, Toyama MH, De Campos Vidal B (2008) Extracellular matrix of porcine pericardium: biochemistry and collagen architecture. J Membr Biol 221(1): 15–25
Cacho F, Elbischger PJ, Rodriguez JF, Doblare M, Holzapfel GA (2007) A constitutive model for fibrous tissues considering collagen fiber crimp. Int J Nonlinear Mech 42(2): 391–402
Campagnola PJ, Loew LM (2003) Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. NatBiotechnol 21(11): 1356–1360
Canham PB, Finlay HM, Dixon JG, Boughner DR, Chen A (1989) Measurements from light and polarised light microscopy of human coronary arteries fixed at distending pressure. Cardiovasc Res 23(11): 973–982
Canham PB, Talman EA, Finlay HM, Dixon JG (1991) Medial collagen organization in human arteries of the heart and brain by polarized light microscopy. Connect Tissue Res 26(1–2): 121–134
Canham PB, Whittaker P, Barwick SE, Schwab ME (1992) Effect of pressure on circumferential order of adventitial collagen in human brain arteries. Can J Physiol Pharmacol 70(2): 296–305
Chambers JM, Cleveland WS, Kleiner B, Tukey PA (1983) Graphical methods for data analysis. Wadsworth & brooks/Cole Publishing Company, Pacific Grove
Dingemans KP, Teeling P, Lagendijk JH, Becker AE (2000) Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec 258(1): 1–14
Dobrin PB (1996) Effect of histologic preparation on the cross-sectional area of arterial rings. J Surg Res 61(2): 413–415
Elbischger PJ, Bischof H, Holzapfel GA, Regitnig P (2005) Computer vision analysis of collagen fiber bundles in the adventitia of human blood vessels. Stud Health Technol Inf 113: 97–129
Elbischger PJ, Bischof H, Regitnig P, Holzapfel GA (2004) Automatic analysis of collagen fiber orientation in the outermost layer of human arteries. Pattern Anal Appl 7(3): 269–284
Et-Taouil K, Schiavi P, Levy BI, Plante GE (2001) Sodium intake, large artery stiffness, and proteoglycans in the spontaneously hypertensive rat. Hypertension 38(5): 1172–1176
Ferdman AG, Yannas IV (1993) Scattering of light from histologic sectionsA: new method for the analysis of connective tissue. J Invest Dermatol 100(5): 710–716
Finlay HM, McCyllough L, Canham PB (1995) Three-dimensional collagen organization of human brain arteries at different transmural pressures. J Vasc Res 32(5): 301–312
Franchi M, Fini M, Quaranta M, De Pasquale V, Raspanti M, Giavaresi G, Ottani V, Ruggeri A (2007) Crimp morphology in relaxed and stretched rat Achilles tendon. J Anat 210(1): 1–7
Greenwald SE, Moore JE Jr, Rachev A, Kane TP, Meister JJ (1997) Experimental investigation of the distribution of residual strains in the artery wall. J Biomech Eng 119(4): 438–444
Han HC, Fung YC (1996) Direct measurement of transverse residual strains in aorta. Am J Physiol 270(2 Pt 2): H750–759
Hansen KA, Weiss JA, Barton JK (2002) Recruitment of tendon crimp with applied tensile strain. J Biomech Eng 124(1): 72–77
Hilbert SL, Sword LC, Batchelder KF, Barrick MK, Ferrans VJ (1996) Simultaneous assessment of bioprosthetic heart valve biomechanical properties and collagen crimp length. J Biomed Mater Res 31(4): 503–509
Jahne B (1993) Spatio-temporal image processing: theory and scientific aplications. Springer, Berlin
Krahn KN, Bouten CV, van Tuijl S, van Zandvoort MA, Merkx M (2006) Fluorescently labeled collagen binding proteins allow specific visualization of collagen in tissues and live cell culture. Anal Biochem 350(2): 177–185
Lanir Y (1983) Constitutive equations for fibrous connective tissues. J Biomech 16(1): 1–12
Magnusson SP, Qvortrup K, Larsen JO, Rosager S, Hanson P, Aagaard P, Krogsgaard M, Kjaer M (2002) Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Biol 21(4): 369–377
Mardia KV, Jupp PE (2000) Directional statistics. Wiley series in probability and statistics. Wiley, Chichester
Megens RT, Reitsma S, Schiffers PH, Hilgers RH, De Mey JG, Slaaf DW, oude Egbrink MG, van Zandvoort MA (2007) Two-photon microscopy of vital murine elastic and muscular arteries. Combined structural and functional imaging with subcellular resolution. J Vasc Res 44(2): 87–98
Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M (2004) Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 58(2): 167–176
Rieppo J, Hallikainen J, Jurvelin JS, Kiviranta I, Helminen HJ, Hyttinen MM (2008) Practical considerations in the use of polarized light microscopy in the analysis of the collagen network in articular cartilage. Microsc Res Tech 71(4): 279–287
Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL (2002) Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J Biomech Eng 124(2): 214–222
Sacks MS, Smith DB, Hiester ED (1997) A small angle light scattering device for planar connective tissue microstructural analysis. Ann Biomed Eng 25(4): 678–689
Smith JFH, Canham PB, Starkey J (1981) Orientation of collagen in the tunica adventitia of the human cerebral artery measured with polarized light and the universal stage. J Ultrastruct Res 77(2): 133–145
Unser M, Aldroubi A, Eden M (1993) B-spline signal processing. Part I. Theory. IEEE Trans Signal Process 41(2): 821–833
Voytik-Harbin SL, Rajwa B, Robinson JP (2001) Three-dimensional imaging of extracellular matrix and extracellular matrix-cell interactions. Methods Cell Biol 63: 583–597
Whittaker P, Canham PB (1991) Demonstration of quantitative fabric analysis of tendon collagen using two-dimensional polarized light microscopy. Matrix 11(1): 56–62
Wicker BK, Hutchens HP, Wu Q, Yeh AT, Humphrey JD (2008) Normal basilar artery structure and biaxial mechanical behaviour. Comput Methods Biomech Biomed Eng 11(5): 539–551
Williams C, Liao J, Joyce EM, Wang B, Leach JB, Sacks MS, Wong JY (2009) Altered structural and mechanical properties in decellularized rabbit carotid arteries. Acta Biomater 5(4): 993–1005
Wolinsky H, Glagov S (1964) Structural basis for the static mechanical properties of the aortic media. Circ Res 14: 400–413
Xia Y, Elder K (2001) Quantification of the graphical details of collagen fibrils in transmission electron micrographs. J Microsc 204(1): 3–16
Young AA, Legrice IJ, Young MA, Smaill BH (1998) Extended confocal microscopy of myocardial laminae and collagen network. J Microsc 192(2): 139–150
Zulliger MA, Fridez P, Stergiopulos N, Hayashi K (2004) A strain energy function for arteries accounting for wall composition and structure. J Biomech 37(7): 989–1000
Acknowledgments
The authors would like to thank Dr. Dimitrios Kontaxakis for helpful discussions on statistical distributions and Dr. Tyler Thacher for proofreading the article. This work was supported by the Swiss National Science Foundation (Grant No. 325230_125445).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Rezakhaniha and A. Agianniotis contributed equally to this work.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Rezakhaniha, R., Agianniotis, A., Schrauwen, J.T.C. et al. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech Model Mechanobiol 11, 461–473 (2012). https://doi.org/10.1007/s10237-011-0325-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-011-0325-z