Abstract
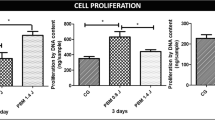
In this study, it was hypothesized that Pluronic F-68 (PLF-68) increases matrix synthesis of osteoarthritis (OA) chondrocytes in addition to its well-documented cell survival effect. To test this hypothesis, rat articular chondrocytes were embedded in agarose discs and were exposed to 5-azacytidine (Aza-C) to induce OA-like alterations. Chondrocytes were then treated with PLF-68 (8 and 12 mg/ml) for 10 days. Aza-C-exposed and PLF-68-untreated chondrocytes and Aza-C-unexposed and PLF-68-untreated chondrocytes were used as negative and positive control groups, respectively. Dynamic hydrostatic pressure (max 0.2 MPa, 0.1 Hz) was applied to discs for 30 min/day (5 days/week). Cell viability, collagen and proteoglycan deposition in discs were determined. Unconfined compression stress relaxation tests were performed to determine peak stress and material parameters of discs—namely spring constants (k 1 and k 2), damping coefficient (η), instantaneous modulus (E 0) and relaxed modulus (E ∞) using Kelvin model to evaluate the functional coherence of the matrix. PLF-68 treatment significantly increased the collagen deposition in discs and viability of OA-like chondrocytes. A dose-dependent increase was also observed for elastic stiffness parameters (k 1, k 2, E 0 and E ∞). Same positive effect of PLF-68 was not observed for proteoglycan deposition. However, dose-dependent increase in η suggests that PLF-68 treatment resulted with the deposition of functional matrix. This is the first study which reports that PLF-68 has also positive effect on collagen synthesis of OA cells. As a conclusion, our results suggest that PLF-68 has a potential for recovery from OA-like alterations, which should be further analyzed.
Similar content being viewed by others
References
Baars DC, Rundell SA, Haut RC (2006) Treatment with the non-ionic surfactant poloxamer P188 reduces DNA fragmentation in cells from bovine chondral explants exposed to injurious unconfined compression. Biomech Model Mechanobiol 5(2–3): 133–139. doi:10.1007/s10237-006-0024-3
Bader DL, Kempson GE, Egan J, Gilbey W, Barrett AJ (1992) The effects of selective matrix degradation on the short-term compressive properties of adult human articular cartilage. Biochim Biophys Acta 1116(2): 147–154
Benya PD, Shaffer JD (1982) Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30(1): 215–224
Blanco FJ, Guitian R, Vázquez-Martul E, de Toro FJ, Galdo F (1998) Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum 41(2): 284–289
Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB (2001) The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res 19(1): 11–17
Farndale RW, Buttle DJ, Barrett AJ (1986) Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883(2): 173–177. doi:10.1016/0304-4165(86)90306-5
Fung YC (1993) Biomechanics: mechanical properties of living tissues. Springer-Verlag, New York, p p 41
Gigout A, Buschmann MD, Jolicoeur M (2009) Chondrocytes cultured in stirred suspension with serum-free medium containing pluronic-68 aggregate and proliferate while maintaining their differentiated phenotype. Tissue Eng Part A 15(8): 2237–2248. doi:10.1089/ten.tea.2008.0256
Hannig J, Zhang D, Canaday DJ, Beckett MA, Astumian RD, Weichselbaum RR, Lee RC (2000) Surfactant sealing of membranes permeabilized by ionizing radiation. Radiat Res 154(2): 171–177
Hansen U, Schünke M, Domm C, Ioannidis N, Hassenpflug J, Gehrke T, Kurz B (2001) Combination of reduced oxygen tension and intermittent hydrostatic pressure: a useful tool in articular cartilage tissue engineering. J Biomech 34(7): 941–949
Hellung-Larsen P, Assaad F, Pankratova S, Saietz BL, Skovgaard LT (2000) Effects of Pluronic F-68 on Tetrahymena cells: protection against chemical and physical stress and prolongation of survival under toxic conditions. J Biotechnol 76(2–3): 185–195
Hidvegi NC, Sales KM, Izadi D, Ong J, Kellam P, Eastwood D, Butler PE (2006) A low temperature method of isolating normal human articular chondrocytes. Osteoarthr Cartil 14(1): 89–93. doi:10.1016/j.joca.2005.08.007
Ho ML, Chang JK, Wu SC, Chung YH, Chen CH, Hung SH, Wang GJ (2006) A novel terminal differentiation model of human articular chondrocytes in three dimensional cultures mimicking chondrocytic changes in osteoarthritis. Cell Biol Int 30(3): 288–294. doi:10.1016/j.cellbi.2005.11.009
Hoemann CD, Sun J, Chrzanowski V, Buschmann MD (2002) A multivalent assay to detect glycosaminoglycan, protein, collagen, RNA, and DNA content in milligram samples of cartilage or hydrogel-based repair cartilage. Anal Biochem 300(1): 1–10. doi:10.1006/abio.2001.5436
Huang CY, Mow VC, Ateshian GA (2001) The role of flow-independent viscoelasticity in the biphasic tensile and compressive responses of articular cartilage. J Biomech Eng 123(5): 410–417. doi:10.1115/1.1392316
Huang CY, Soltz MA, Kopacz M, Mow VC, Ateshian GA (2003) Experimental verification of the roles of intrinsic matrix viscoelasticity and tension-compression nonlinearity in the biphasic response of cartilage. J Biomech Eng 125(1): 84–93. doi:10.1115/1.1531656
Karna E, Miltyk W, Pałka JA, Jarzabek K, Wołczyński S (2006) Hyaluronic acid counteracts interleukin-1-induced inhibition of collagen biosynthesis in cultured human chondrocytes. Pharmacol Res 54(4): 275–281. doi:10.1016/j.phrs.2006.06.002
Katakam M, Bell LN, Banga AK (1995) Effect of surfactants on the physical stability of recombinant human growth hormone. J Pharm Sci 84(6): 713–716. doi:10.1002/jps.2600840609
Koppenol S (2008) Physical considerations in protein and peptide stability. In: McNally EJ, Hastedt JE (eds) Protein formulation and delivery, drugs and pharmaceutical sciences, vol 175, 2nd edn. Informa Healthcare Inc, USA, pp 43–73
Kühn K, D’Lima DD, Hashimoto S, Lotz M (2004) Cell death in cartilage. Osteoarthr Cartil 12(1): 1–16. doi:10.1016/j.joca.2003.09.015
Lee RC, Hannig J, Matthews KL, Myerov A, Chen C-T (1999) Pharmaceutical therapies for sealing of permeabilized cell membranes in electrical injuries. Ann N Y Acad Sci 888: 266–273
Leipzig ND, Athanasiou KA (2005) Unconfined creep compression of chondrocytes. J Biomech 38(1): 77–85. doi:10.1016/j.jbiomech.2004.03.013
Lorenz H, Richter W (2006) Osteoarthritis: cellular and molecular changes in degenerating cartilage. Prog Histochem Cytochem 40(3): 135–163. doi:10.1016/j.proghi.2006.02.003
Marks JD, Pan C-Y, Bushell T, Cromie W, Lee RC (2001) Amphiphilic, tri-block copolymers provide potent, membrane-targeted neuroprotection. FASEB J 15(6): 1107–1109. doi:10.1096/fj.00-0547fje
Martinez V, Corsini E, Mitjans M, Pinazo A, Vinardell MP (2006) Evaluation of eye and skin irritation of arginine-derivative surfactants using different in vitro endpoints as alternatives to the in vivo assays. Toxicol Lett 164(3): 259–267. doi:10.1016/j.toxlet.2006.01.005
Maskarinec SA, Hannig J, Lee RC, Lee KYC (2002) Direct observation of poloxamer 188 insertion into lipid monolayers. Biophys J 82(3): 1453–1459. doi:10.1016/S0006-3495(02)75499-4
Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT (2003) Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng 9(4): 597–611. doi:10.1089/107632703768247304
Mauck RL, Soltz MA, Wang CCB, Wong DD, Chao P-HG, Valhmu WB, Hung CT, Ateshian GA (2000) Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 122(3): 252–260
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2): 55–63
Mouw JK, Case ND, Guldberg RE, Plaas AHK, Levenston ME (2005) Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthr Cartil 13(9): 828–836. doi:10.1016/j.joca.2005.04.020
Na K, Park JH, Kim SW, Sun BK, Woo DG, Chung HM, Park KH (2006) Delivery of dexamethasone, ascorbate, and growth factor (TGF beta-3) in thermo-reversible hydrogel constructs embedded with rabbit chondrocytes. Biomaterials 27(35): 5951–5957. doi:10.1016/j.biomaterials.2006.08.012
Nesic D, Whiteside R, Brittberg M, Wendt D, Martin I, Mainil-Varlet P (2006) Cartilage tissue engineering for degenerative joint disease. Adv Drug Deliv Rev 58(2): 300–322. doi:10.1016/j.addr.2006.01.012
O’Hara BP, Urban JP, Maroudas A (1990) Influence of cyclic loading on the nutrition of articular cartilage. Ann Rheum Dis 49(7): 536–539. doi:10.1136/ard.49.7.536
Padanilam JT, Bischof JC, Lee RC, Cravalho EG, Tompkins RG, Yarmush ML, Toner M (1994) Effectiveness of poloxamer 188 in arresting calcein leakage from thermally damaged isolated skeletal muscle cells. Ann N Y Acad Sci 720: 111–123. doi:10.1111/j.1749-6632.1994.tb30439.x
Paêka JA, Karna E, Miltyk W (1997) Fibroblast chemotaxis and prolidase activity modulation by insulin-like growth factor II and mannose 6-phosphate. Mol Cell Biochem 168(1–2): 177–183. doi:10.1023/A:1006842315499
Phillips DM, Haut RC (2004) The use of a non-ionic surfactant (P188) to save chondrocytes from necrosis following impact loading of chondral explants. J Orthop Res 22(5): 1135–1142. doi:10.1016/j.orthres.2004.02.002
Poole AR (2003) What type of cartilage repair are we attempting to attain. J Bone Joint Surg Am 85-A(Suppl 2): 40–44
Rundell SA, Baars DC, Phillips DM, Haut RC (2005) The limitation of acute necrosis in retro-patellar cartilage after a severe blunt impact to the in vivo rabbit patello-femoral joint. J Orthop Res 23(6): 1363–1369. doi:10.1016/j.orthres.2005.06.001.1100230618
Sandell LJ, Aigner T (2001) Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res 3(2): 107–113. doi:10.1186/ar148
Sharma G, Saxena RK, Mishra P (2007) Differential effects of cyclic and static pressure on biochemical and morphological properties of chondrocytes from articular cartilage. Clin Biomech 22(2): 248–255. doi:10.1016/j.clinbiomech.2006.09.008
Toyoda T, Seedhom BB, Yao JQ, Kirkham J, Brookes S, Bonass WA (2003) Hydrostatic pressure modulates proteoglycan metabolism in chondrocytes seeded in agarose. Arthritis Rheum 48(10): 2865–2872. doi:10.1002/art.11250
Vrahas MS, Mithoefer K, Joseph D (2004) The long-term effects of articular impaction. Clin Orthop Relat Res 423: 40–43. doi:10.1097/01.blo.0000133567.28491.7d
Zuscik MJ, Baden JF, Wu Q, Sheu TJ, Schwarz EM, Drissi H, O’Keefe RJ, Puzas JE, Rosier RN (2004) 5-azacytidine alters TGF-beta and BMP signaling and induces maturation in articular chondrocytes. J Cell Biochem 92(2): 316–331. doi:10.1002/jcb.20050
Author information
Authors and Affiliations
Corresponding author
Additional information
The poster presentation of the work was performed at the International Symposium on Biotechnology: Developments and Trends, Middle East Technical University, Ankara, Turkey, September 2009.
Rights and permissions
About this article
Cite this article
Kavas, A., Özdemir, M., Gürses, S. et al. In vitro investigation and biomechanical modeling of the effects of PLF-68 on osteoarthritis in a three-dimensional model. Biomech Model Mechanobiol 10, 641–650 (2011). https://doi.org/10.1007/s10237-010-0262-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-010-0262-2