Abstract
South Africa has a growing population, a relatively dry climate, and abundant mining activity, all of which increase the importance of water management. The Mooiplaas Dolomite Quarry, south east of Pretoria, has been mining metallurgical grade dolomite since 1969, within the productive karst aquifers of the Malmani Subgroup, Transvaal Supergroup. This study was conducted to elucidate the flow of water around the site, including the mine water and groundwater. The site was investigated by sampling precipitation, surface water, groundwater, and mine water for hydrochemical and stable isotope analysis from 2011 to 2017, totalling over 400 samples. Levels of nitrate in groundwater and mine water were marginally above drinking water limits, from explosives residues, and ammonia in the nearby Hennops River was unacceptably high due to municipal sewage outfalls, but otherwise, water quality was very good. Alkalinity from rock weathering, aided by crushing of dolomite, was the main control on water chemistry. Combined analysis of dissolved matter (TDS, nitrate, Mg, etc.) suggested that the dewatering of the mine and resultant recharge from the slimes dams caused an aerated zone of groundwater, which mixed with regional groundwater flowing beneath the site. Stable isotopes, with an evaporated signature from the mine open water bodies, also showed how mine operations cause recharge to groundwater and subsequent seepage back into the pit lakes. The mine appears not to contaminate the regional groundwater; however, mine designs should avoid situations where process water flows via groundwater back into pits, causing excessive dewatering costs.
摘要
南非人口不断增长,气候相对干燥,且采矿活动丰富,都增加了水管理的重要性。位于比勒陀利亚东南部的穆伊普拉斯白云石采石场自1969年以来一直在位于马尔马尼亚群Transvaal超群的岩溶含水层中,开采冶金级白云石。本研究旨在阐明该地区的水流情况,包括矿井水和地下水。从 2011年到 2017年,通过对降水、地表水、地下水和矿井水取样进行水化学和稳定同位素分析,对该矿进行了调查,共采集了400多个样本。地下水和矿井水中的硝酸盐含量略高于饮用水限值,这是爆炸物残留造成的,而附近Hennops 河中的氨含量因市政污水排放而过高,但除此之外,水质非常好。由于白云石的破碎,岩石风化产生的碱度是控制水化学的主要因素。对溶解物质(TDS、硝酸盐、镁等)的综合分析表明,矿井的排水和来自泥浆坝的补给导致了一个充气的地下水区,该区域与流经该地区的区域地下水混合。稳定同位素还显示出蒸发后的矿井开放水体特征,显示了采矿作业如何导致地下水补给,随后又如何渗入矿坑湖泊。该矿井似乎没有污染区域地下水;但是,矿井设计应避免加工水通过地下水流回矿坑而造成过高的排水成本.
Zusammenfassung
Südafrika hat eine wachsende Bevölkerung, ein relativ trockenes Klima und rege Bergbautätigkeit, was die Bedeutung des Wassermanagements erhöht. Im Dolomitsteinbruch Mooiplaas, südöstlich von Pretoria, wird seit 1969 metallurgisch hochwertiger Dolomit in den ergiebigen Karstgrundwasserleitern der Malmani-Untergruppe der Transvaal-Supergruppe abgebaut. Diese Studie wurde durchgeführt, um den Wasserfluss in der Umgebung des Standorts, einschließlich des Grubenwassers und des Grundwassers, zu erforschen. Zur Untersuchung des Standorts wurden von 2011 bis 2017 Niederschlags-, Oberflächen-, Grund- und Grubenwasserproben für hydrochemische Analysen und Analysen stabiler Isotope entnommen, insgesamt über 400 Proben. Die Nitratwerte im Grund- und Grubenwasser lagen aufgrund von Sprengstoffrückständen geringfügig über den Trinkwassergrenzwerten und der Ammoniakgehalt im nahegelegenen Hennops River war aufgrund kommunaler Abwassereinleitungen unannehmbar hoch, aber ansonsten war die Wasserqualität sehr gut. Die Alkalinität aus der Gesteinsverwitterung, die durch die Zerkleinerung des Dolomits begünstigt wird, war der wichtigste Faktor für die Wasserchemie. Die kombinierte Analyse der gelösten Stoffe (TDS, Nitrat, Mg usw.) deutet darauf hin, dass die Entwässerung des Tagbaus und die daraus resultierende Anreicherung aus den Schlammteichen eine kohlensäurehaltige Grundwasserzone verursachte, die sich mit dem regionalen Grundwasser, das unter dem Gelände fließt, vermischte. Stabile Isotope mit einer Verdunstungssignatur aus den offenen Wasserkörpern des Bergwerks zeigten auch, wie der Betrieb des Bergwerks die Anreicherung des Grundwassers und die anschließende Rückversickerung in die Tagbauseen verursacht. Der Tagbau scheint das regionale Grundwasser nicht zu verunreinigen; bei der Planung von Tagbauen sollten jedoch vermieden werden, dass Betriebswasser über das Grundwasser in die Tagbaue zurückfließt und übermäßige Entwässerungskosten verursacht.
Resumen
Sudáfrica tiene una creciente población, un clima relativamente seco y una intensa actividad minera, lo que aumenta la importancia de la gestión del agua. La cantera dolomítica de Mooiplaas, al sureste de Pretoria, ha estado extrayendo dolomita de calidad metalúrgica desde 1969, dentro de los acuíferos kársticos productivos del Subgrupo Malmani, del Supergrupo Transvaal. Este estudio se realizó para dilucidar el flujo de agua alrededor de la cantera, incluyendo el agua de la mina y el agua subterránea. El sitio fue investigado mediante el muestreo de agua de precipitación, agua superficial, agua subterránea y agua de la mina para análisis hidroquímicos y de isotópos estables desde 2011 hasta 2017, totalizando más de 400 muestras. Los niveles de nitrato en el agua subterránea y de mina estaban ligeramente por encima de los límites establecidos para agua potable, provenientes de residuos de explosivos, mientras que los niveles de amonio en el cercano río Hennops era inaceptablemente alto debido a los vertidos de aguas residuales municipales, pero por lo demás, la calidad del agua era muy buena. La alcalinidad resultante de la meteorización de rocas, ayudada por la trituración de dolomita, fue el principal control sobre la química del agua. El análisis combinado de materia disuelta (TDS, nitrato, Mg, etc.) sugirió que el desagüe de la mina y la recarga resultante de las presas de lodos causaron una zona aireada de agua subterránea, que se mezclaba con el agua subterránea regional que fluía debajo de la cantera. Los isótopos estables, que indican fuerte evaporación de los cuerpos de agua superficiales de la mina, también mostraron cómo las operaciones mineras causan la recarga al agua subterránea y posterior filtración de vuelta a los lagos de la mina. La mina parece no contaminar el agua subterránea regional; sin embargo, los diseños de las minas deberían evitar situaciones donde el agua de proceso fluya a través del agua subterránea en el entorno de la mina, causando costos excesivos de desagüe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water resources globally are under threat from the growing human population (Kåresdotter et al. 2022; Kummu et al. 2010). The global population of approximately 8 billion people not only consume fresh water, but also produce pollution that taints many freshwater resources (Simonovic 2002). In addition to these direct effects, indirect effects of ecological destruction and climate change are threatening water resources and the ability of humans to provide for their needs (Nhemachena et al. 2020).
Groundwater occurs in much larger quantities than surface water (Poeter et al. 2020), but a lot of this is too deep to easily exploit and often too salty for most uses (Gleeson et al. 2015). Despite this, groundwater supplies many billions of people and much of the agricultural and industrial activity of humans (Jasechko and Perrone 2021). Importantly, surface water resources are often absent, polluted, or not easily stored or distributed, so groundwater often provides a better, or the only water supply. It is estimated that 50% of the global urban population rely on groundwater for their personal use (UNESCO 2022).
Karst aquifers are some of the strongest groundwater resources, providing major water supplies to human activities and the environment in many parts of the world (Doveri et al. 2021; Gao et al. 2011; Schrader and Winde 2015). South Africa has relatively minimal carbonate rock, but where it does occur, aquifers of regional significance are found, in particular the dolomites of the Malmani Subgroup that occur from Pretoria westwards to the border with Botswana (Meyer 2014).
South Africa is a relatively dry land, with a mean annual precipitation of around 450 mm/a (Dent et al. 1989), which is not much over half the global figure of 750 mm/a (Oki and Kanae 2006). Groundwater use is not well measured or managed in South Africa (Pietersen 2006), but estimates are that about 15% of the total amount of water used by humans is groundwater, two-thirds of which is for irrigated agriculture, with nearly 300 towns fully dependent on groundwater (Knüppe 2011).
Water quality is as important as the amount of water available. Most human activities have the ability to impact water quality negatively, including domestic activities (Baloyi and Diamond 2019), urban areas (Germishuys and Diamond 2022), and mining (Winde 2013). Both underground and open pit mines affect water quality, with the nature of the pollution dependent on the mineralogy, water chemistry, rock structure, climate, and other environmental factors (Nordstrom 2011). The effects on groundwater quality from mining have been studied widely (Eary 1999), including in karst environments (Gao et al. 2011). Stable isotopes of water (hydrogen and oxygen) or dissolved constituents (such as NO3− or SO42−) can be used as tracers of water flow, or to identify the sources of the water or dissolved matter (Cook 2020).
This study made use of hydrochemistry and the stable isotopes of water to help understand the flow of water in and around the Mooiplaas Dolomite Quarry. We evaluated the effect of the quarry on the groundwater of the area and suggest ways to improve site water management, as well as ideas for further study.
Site Description
Location
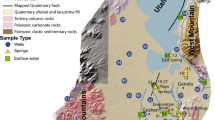
The Mooiplaas Dolomite Quarry has been mining metallurgical and aggregate grade dolomite since 1969. It is currently operated by Pretoria Portland Cement and is located on the south-western edge of Pretoria in Gauteng Province, South Africa (Fig. 1). Gauteng is the smallest of nine provinces in South Africa, but the most populous at about 16 million (Wikipedia 2022), and is mostly covered by the urban areas associated with Johannesburg in the south and Pretoria in the north.
Geology
The city of Pretoria, located 15 km north-east of the quarry, is underlain by the Pretoria Group, a lightly metamorphosed volcano-sedimentary sequence. This is underlain by the Chuniespoort Group, which includes the dolomites and cherty-dolomites of the Malmani Subgroup, all part of the Transvaal Supergroup, a relatively undeformed Archean to Proterozoic platform sequence forming part of the Kaapvaal Craton (Eriksson et al. 2006).
Mooiplaas mines mainly the Lyttleton Formation, a 100–150 m thick, chert-poor formation, which lies above the Monte Christo Formation and below the Eccles Formation, both of which are chert-rich, and all of which are part of the Malmani Subgroup. The final product, after milling, of metallurgical grade dolomite contains less than 3.5% SiO2 and 0.5% Al2O3 (Page and Du Plessis 1986). The geology of the region dips gently northwards at 10–15°, a tilt caused by crustal subsidence after the intrusion of the Bushveld Igneous Complex around 2 Ga ago. Faults and mafic intrusions complicate the geology somewhat (Figs. 1 and 2).
Climate
The climate of the region is subtropical, with moderate temperature seasonality, but strong rainfall seasonality. Winters (May to July) have cold nights with minimums around 5 °C and warm days with maximums around 20 °C and negligible rainfall, whereas summer lasts from October to March, when temperatures are similarly 16 °C and 28 °C and monthly mean rainfall is around 100 mm (Fig. 3) (CSAG 2022). Total mean annual rainfall is about 700 mm. Winds are light, except for brief downdraughts associated with summer thunderstorms (SAWS 2021).
Climate of the region (CSAG 2022)
Geomorphology
Gauteng Province is a relatively flat area with gentle topography of rolling hills or low rocky ridges, typically up to 200 m above the surrounding plains. The whole region, however, is at high elevation, ranging from 1200 to 1800 masl.
Hydrology
The continental watershed runs east-west through central Johannesburg, with southward-flowing streams entering the Vaal River before joining the Gariep (Orange) River and flowing west to the Atlantic Ocean. Northward-flowing streams enter the Crocodile River before joining the Limpopo and flowing north and then east to the Indian Ocean. The Hennops River that flows past Mooiplaas Quarry is part of the latter catchment (Fig. 1).
Hydrogeology
The Malmani Subgroup dolomites are mildly karstified, with minimal surface water features, common sinkholes and dolines especially in the chert-rich formations, pinnacles, and grykes developed to several metres depth, and highly porous gryke infill called WAD (weathered altered dolomite) (Dippenaar et al. 2019). They form a high yielding aquifer that is utilized for agriculture, domestic, and public water supply (Meyer 2014). Borehole yields are typically over 5 L/s (DWAF 1999). Many springs emerge from the dolomites where they abut less permeable formations, some of which are today used for Pretoria’s water supply (Dippenaar et al. 2019), and in the past were part of the reason our ancestors inhabited the Cradle of Humankind World Heritage Site, north-west of Johannesburg (Hobbs and De Meillon 2017).
The large gold mining operations in the Witwatersrand Supergroup near Johannesburg had to dewater tremendous volumes from the dolomites to allow mining, and now that much of the gold mining is over, water levels are recovering, and springs are starting to flow again, although often with poor water quality due to acid mine drainage (Schrader et al. 2014). Water quality in the dolomite aquifers is generally excellent, but effects from mining, agriculture and urban areas, often via polluted rivers, are likely to be increasing (Meyer 2014).
Methods
Field Work
Water samples were taken of rain, surface water, and groundwater at 20 locations in and around Mooiplaas Quarry from 2011 to 2017, totalling 407 samples.
Precipitation
Rain (and hail) was collected from December 2016 until May 2017 in Monument Park, 15 km to the east of Mooiplaas, totalling seven samples (two were taken in February—see Table 1). A standard plastic rain gauge was used to collect all precipitation, the amount of which was noted daily, after which all the water was placed into a larger bottle to create a composite sample for each month. Each month’s sample was analysed for hydrogen and oxygen stable isotopes and select samples were analysed for dissolved matter.
Surface Water
The only natural surface water locations sampled were two sites on the Hennops River, upstream and downstream of the quarry (HRU and HRD), totalling 49 samples.
Groundwater
The distinction between surface water and groundwater is not simple at a quarry. For example, the pit lakes are open to the air and so qualify as surface water but are at the level of the water table and represent groundwater. Nonetheless, 17 locations were sampled for surface water/groundwater, of which six are boreholes, three are seeps and eight are various points in the water cycle of the quarry, including the pit lakes, various slurry or slimes dams, and sumps or inlets, totalling 352 samples.
Laboratory Work
Hydrochemistry
Analysis was performed at Aquatico Laboratories in Pretoria. Electrical conductivity (EC) and pH were measured potentiometrically with an autosampler, where a Mode d’Emploi 2-pole conductivity cell was used for EC and a Red Rod pH probe was used for pH. TDS was measured gravimetrically after filtering out > 2 μm suspended solids and then evaporation in a pre-weighed dish, according to APHA (2005) procedures. Alkalinity, Cl, SO4, PO4, N03, and NH4 were analysed using a KoneLab automated spectrophotometer, and Br using a desktop Hach spectrophotometer (APHA 2005). Na, Mg, K, and Ca were analysed with a Perkin Elmer Optima 7300DV or Optima 8300 ICP-OES (inductively coupled plasma optical emission spectrometer).
Stable Isotopes
Analysis was performed at iThemba Labs in Johannesburg using a Los Gatos Research DLT-100, according to the procedures outlined by the IAEA (2009). This instrument fills a cavity with a vapourised sample and then fires a laser into the cavity with high reflectivity mirrors, generating a path length of kilometres, such that water molecules of different isotopic composition cause different degrees of absorption.
Data Correction and Validation
Hydrochemistry
In addition to laboratory calibration procedures, blanks (distilled water) and duplicates of field samples were submitted blind (unknown to the laboratory) with every batch of samples. The relative percent difference (RPD) for analytes was calculated for duplicate samples. Over 80% of the duplicate analyses had RPD < 10%. In addition, the charge balance was calculated, comparing the sum of cations to the sum of anions as milliequivalents, and it was found the RPD for charge balance was < 10% for all samples.
Stable Isotopes
Laboratory standards were inserted with every batch of samples, to allow correction to the international standard, SMOW (Standard Mean Ocean Water). In addition, blind duplicates were inserted with the samples to provide a check on the laboratory’s precision. According to iThemba Labs, their analytical precision is approximately 1.5‰ for δ2H and 0.5‰ for δ18O. Analysis of duplicates showed that RPD was < 10% for all duplicates.
Results
Hydrochemistry
Water Quality Assessment
Table 2 shows the mean values for each analyte at each sample location, and overall for the study. Shading indicates where values exceed ideal, acceptable, and maximum allowable drinking water quality guidelines for South Africa (SABS 2001). As can be seen from the table, the water quality around Mooiplaas is generally very good. The major exceptions are ammonia, which is moderately elevated in the Hennops River, the sump, and the two exploration boreholes, and nitrate which is slightly elevated in a few of the sample locations.
The ammonia in the Hennops River is likely to be from wastewater from sewage treatment works that discharge into the River (Rimayi et al. 2019). The presence of ammonia in the exploration boreholes is discussed later. The elevated nitrate, which is found in most of the mine water samples, is probably from explosives used for mining (Nilsson and Widerlund 2017).
Hydrochemical Trends
Several scatter plots have been drawn (Figs. 4, 5, 6, 7) showing the most interesting hydrochemical patterns. In Fig. 4, a very clear positive correlation exists between alkalinity and total dissolved solids (TDS). Alkalinity at the circumneutral pH’s of this site is largely bicarbonate ions (HCO3−), which form during the process of rock weathering. In this process, atmospheric or soil CO2 dissolves into rain or soil water, forming a solution of weak carbonic acid (H2CO3), which dissociates into hydrogen and bicarbonate ions, the H+ then being exchanged for a metal cation (e.g. K+) in a mineral. In addition to this process, which operates everywhere at the earth’s surface where there is rain, the study area is underlain by dolomite. The dissolution of dolomite (CaMgCO3) releases CO32− ions into the water, most of which will be converted to bicarbonate from hydrogen ions available in the mildly acidic rain/soil water. By this mechanism, natural waters increase in metal cations and bicarbonate ions over time. The strong correlation of alkalinity with TDS proves the control that rock weathering has on water quality, and the gradient of 0.57 seems to show that nearly 60% of the dissolved matter can be accounted for by the alkalinity. In actual fact, because the alkalinity is reported as CaCO3, only 60% of this mass is actually carbonate or bicarbonate. As 60% of the 0.57 correlation is approximately 0.4, this means the alkalinity actually accounts for approximately 40% of the TDS (Table 2 and Fig. 4).
Chloride concentrations plotted against TDS (total dissolved solids). The low gradient shows that Cl has a lesser role in the dissolved water quality components than alkalinity. This is to be expected for inland locations, far from the sea. Relatively high Cl samples from the Hennops River are likely from sewage spills from municipal waste water treatment works
In contrast, the other major anion, chloride, which also correlates well with TDS, is at a much lower slope of 0.24 (Fig. 5). This slope would be even lower, if not for five outlier samples from the Hennops River, probably representing sewage spills of highly evaporated wastewater with high salt contents. The lower chloride content is typical of inland locations, such as Pretoria, where marine aerosol input is minimal (Van Wyk et al. 2011). What is also noticeable from Fig. 5 is the distinctly higher chloride contents of the Hennops River, compared to the mine water. This is because the mine area water chemistry is more strongly influenced by rock dissolution, encouraged by the blasting and crushing of dolomite.
For the cations, Ca and Mg are the dominant ions (see Table 2), which is typical of inland settings where dissolution of dolomite dominates over marine aerosol. Further evidence for dolomite dissolution being a strong control on the hydrochemistry is the dolomite saturation indices, as calculated using PHREEQC (Table 2). These mostly reveal the water to be supersaturated (SI dol > 0) in dolomite. The lowest groundwater or mine water SI dol values are found in the exploration boreholes, suggesting that the natural groundwater is less saturated in dolomite.
Figure 6 shows the nitrate and ammonia concentrations. There is a strong contrast between the Hennops River samples where ammonia dominates, and virtually all of the other samples, where nitrate dominates. The likely reasons for the pattern in this study are twofold. First, the aerated state of groundwater in and around the mine is to be expected from the quarrying operations, where groundwater is being pumped out and recharged and generally circulated strongly through the pit lakes, the plant, various slurry dams, and so on. Second, the Hennops River receives treated and untreated sewage with a high organic matter content, which translates into a high COD (chemical oxygen demand; Rimayi et al. 2019). This decaying organic matter consumes the available oxygen in the water, causing the reduced form of nitrogen (ammonia) to dominate over the oxidised form (nitrate).
The final hydrochemistry scatter plot, Fig. 7, shows magnesium plotted against TDS. Here there is substantial separation of samples and clear grouping of certain locations, as well as distinctly different trends between the Hennops River, mine water, and groundwater. The mine water circulates in the slurry and slimes dams, the plant inlet, and the pit lakes, while the groundwater was sampled from boreholes and seeps. Overall, both the mine water and groundwater show much higher Mg values than the Hennops River. This is likely due to the blasting and crushing of dolomite at the mine, which accelerates dolomite dissolution and the release of Mg into this water. However, even within the mine area, the groundwater has a lower Mg vs TDS trend than the mine water, which is more directly exposed to the broken-up dolomite. The slurry dams have the highest Mg contents, reflecting their position downstream of the plant and thus the highest exposure to fine dolomite particles.
However, the Mg and TDS levels are fairly low, still being within acceptable drinking water limits. This is partly due to the way in which dolomite dissolution is controlled by acidity. Once the acidity of the water has been used up (the mean pH for this study was 8), dolomite dissolution is negligible. This is confirmed by the high dolomite saturation indices, showing the water to be supersaturated in Ca-Mg-CO3. The low TDS may also be partly due to the way in which the mine water interacts with the local groundwater and is diluted by the local to regional groundwater flowing through the site.
Stable Isotopes
The hydrogen and oxygen isotope results are given in Table 3 and plotted in Fig. 8. The rain isotope composition ranges from about − 70‰ δ2H and − 10‰ δ18O to almost + 10‰ δ2H and 0‰ δ18O. The LMWL (local meteoric water line) was calculated using these points and the reduced major axis form of regression, where both x and y are treated as independent variables, giving the result of δ2H = 8.3δ18O + 12.2. This line is slightly steeper than the GMWL (global meteoric water line; Craig 1961). Many LMWLs are at a gentler gradient than the GMWL (Diamond 2022), but some areas do have LMWLs with gradients around 8; the line calculated for this study is very close to that from Meyer (2014), who calculated a Pretoria LMWL of δ2H = 8δ18O + 11.8.
Stable isotope composition of water samples from the study. The low gradient trend for all except the rain samples, reveals that evaporation is acting on much of the water around the mine site, with the least affected being the exploration boreholes (possibly reflecting natural groundwater of the area), through to the most evaporated samples in the slimes dam
Two precipitation samples are outliers. The upper outlier is for February 2017, during which there was precipitation in 25 of the 28 days, with a total of only 67 mm (Weather and Climate 2023a). These low rainfall events tend to suffer from raindrop evaporation as the rain falls to the ground, thereby generating more positive delta values. The lower outlier is from May 2017, during which there was a single rainfall event over 3 days, producing only 5 mm of rain, but in which temperatures plummeted 10 °C below the norm to 8 °C as a cold front moved across the country (Weather and Climate 2023b). This resulted in an unusually isotopically negative sample. Despite these outliers, the MWL for this study runs through the remaining five samples (one is somewhat obscured by the other data) convincingly.
All the samples except for the rainwater also plot along an excellent regression line (r = 0.98) with the equation δ2H = 4.5δ18O−3.5. This line is at a lower gradient than the meteoric water lines and is typical of a local evaporation line (LEL). The groundwater samples tend to plot at the lower end of the line, and the lowest samples are in fact the two exploration boreholes. These are located to the west of the mining area and are the furthest removed from the pits, crushing plant, and various slurry dams, and so are least involved with the disturbed mine area. As such, they represent relatively undisturbed “natural” groundwater in terms of isotopic composition. It therefore makes sense that they plot close to the LMWL. The groundwater samples with the highest δ values are the two pit wall seeps, where evaporation has acted on the water prior to sampling, possibly as the water drips down the exposed rock faces of the open pits. The G01 and G12 borehole samples plot close to the LWML, but with some tendency towards the right, suggesting mild evaporative effects.
In contrast to the groundwater, the mine water in the various pits and slurry dams has a moderate to highly evaporated isotope signature, plotting furthest from the LWML. This shows that much water is lost to evaporation during the quarrying process. It is also possible that the cause of the evaporated signature for the pit wall seeps (WWS and EWS) is not the final stages of dripping down the exposed pit walls, but rather the local groundwater mixing with some of the mine water. This would happen when water from the highly evaporated slurry dams infiltrates the ground and recharges the water table, and then mixes with the groundwater.
Discussion
The Mooiplaas dolomite quarry affects the local groundwater in several ways. The nitrate levels show that explosives residues dissolve into the groundwater. This nitrogen is almost all in the oxidized state (nitrate), with virtually no ammonia detected, indicating that the groundwater is well oxygenated by the mining activities. This happens through the dewatering of the east and west pits and seepage, re-infiltration, and recharge from the slurry and slimes dams and other ponds (see Fig. 9).
Conceptual diagram of water flows around the Mooiplaas Dolomite Quarry. Groundwater is indicated with dashed lines and evaporation with dots. The exploration boreholes appear to be unimpacted by the mine activity, but may have urban pollution, accounting for their high ammonium levels. The slurry and slimes dams recharge groundwater, which then discharges via the pit wall seeps. This is detected with evaporated stable isotope signatures and high nitrate levels in the pit wall seeps
In contrast, the Hennops River shows high ammonia levels and negligible nitrate. This is due to the discharge of treated and untreated sewage, containing high organic matter contents, into the river, causing high COD and rendering the river water anoxic (Rimayi et al. 2019).
The only two sample sites with significant ammonia, other than the Hennops River, are the exploration boreholes, which, as stated earlier, lie to the west of the active mining area (Fig. 9). The presence of ammonia in these boreholes could result from several factors. First, the informal settlements that occur north of the mining area and at a higher elevation (Fig. 1) have inadequate sanitation, so human waste, rich in ammonia, could be leaching into the ground. In addition, cows and goats are kept and their waste may contribute to the ammonia load. Also, since the exploration boreholes are removed from the most active mining area, they are less likely to receive recently pumped and oxygenated water, keeping the ammonia stable. The low nitrate levels in the groundwater in these two boreholes show that they are uninfluenced by the mining operations.
The highest nitrate levels were found in the slurry dams (mainly SYD & SSD), and in the two wall seeps in the east and west pits (EWS & WWS). As nitrates are extremely soluble, residual explosives from years ago would likely have been leached from the old benches and blast holes above the wall seeps, so the presence of high nitrate in these wall seeps suggests the groundwater being discharged at the wall seeps is being recharged by seepage from the slurry dams upslope to the north.
Crushing of dolomite, creating high surface area, enhances the release of Mg by weathering. This is revealed by comparing the two exploration boreholes, averaging 13 and 37 mg/L Mg2+, against the mine water, with an average of ≈ 60 mg/L. When the Mg-enriched mine water mixes with regional groundwater, the resultant groundwater beneath the mine has intermediate Mg values, averaging 50 mg/L.
The stable isotope results show some remarkable patterns. All the groundwater and mine water samples sit along a LEL; each sample site tends to cluster quite well, and the clusters are fairly well ordered. The order of sample sites, from least to most evaporated, is shown in Fig. 10. As with the Mg data, the two exploration boreholes seem to be the closest to natural groundwater, with isotope compositions very similar to rain water and close to the local and global meteoric water lines. These are followed by the west pit seep, which is on the south-western side of the west pit and furthest from the plant and various dams, and so is likely to be discharging relatively natural groundwater. Then come two boreholes, G01 and G12 and the west pit lake. Note that at this point, most of the samples are groundwater. The next group has more mine water samples, being the east pit lake, the plant inlet, and the fish dam. The last main group contains the west pit wall seep, the MSD slurry dam, and the slurry dam. The groundwater sample here, WWS, could be experiencing dramatic evaporation immediately prior to sampling, as the water trickles over rock slabs, but is also likely influenced by the evaporated water from the slurry dams upgradient (to the north), which recharge the groundwater. In a similar way, the east pit wall seep also shows a very evaporated isotope signature, and finally the slimes dam, which is the last point in the mine water cycle before that water recharges the groundwater.
Increasingly evaporated stable isotope values for sample sites (see Fig. 2). The two exploration boreholes, west of the active mining area, are most similar to meteoric water, followed by mostly groundwater samples and then mine water samples
Conclusion
The main control on water chemistry at Mooiplaas is alkalinity. In this karstic setting, most of the alkalinity is from dissolution of carbonate minerals, mostly dolomite, in the Malmani Subgroup, though weathering of silicates and other minerals also contributes. Ca and Mg are the main cations, also due to the dolomite-dominated geology and the distance from the coast, where Na tends to dominate.
Ammonia is elevated in the Hennops River (HRU & HRD) and the exploration boreholes (EBN & EBS), most likely due to sewage discharged into the former and poor sanitation in Atteridgeville in the latter. Nitrate is elevated in most of the mine water and groundwater samples, from explosive residues. Aside from these moderate ammonia and nitrate levels, the groundwater quality is generally good; however, trace element and bacterial analyses were not performed.
The presence of nitrate and absence of ammonia in the mine water and boreholes within the mining area is evidence of well-aerated groundwater, caused by dewatering, recharge, and active circulation of surface water and groundwater in the mine area. Higher TDS levels in all but the exploration boreholes suggests evaporative concentration, further supporting this model. In contrast, the two exploration boreholes have higher ammonia, indicating less aeration, and lower TDS, indicating less evaporation. This groundwater possibly reflects the regional groundwater condition, although the high ammonia is probably only a local effect from the nearby township and informal settlement.
There appears to be a mixing relationship between the mine water and groundwater based on dissolved Mg trends, as the actively circulated mine water (pits, dams, etc.) mixes with the regional groundwater flowing towards and beneath the mine area. The limited increase in TDS is also evidence for only minor evaporative enrichment due to a sustained regional flow of groundwater, preventing excessive salinisation of the site.
Stable isotopes reveal a strong evaporation trend acting on all of the mine water. The least evaporated samples are the two exploration boreholes, again suggesting these reflect regional groundwater conditions. From there, the various samples progressively show more evaporation, ending with the slurry and slimes dams. The moderately evaporated isotope composition of the east and west pit wall seeps (EWS & WWS) suggest recharge or leakage from the slimes or slurry dams (SSD & SYD), mixed with groundwater, is discharging into the east and west pits. High nitrate levels in the EWS and WWS corroborate this.
The quarry does not appear to contaminate the regional groundwater, although analysis of microorganisms and trace elements, such as Zn and Cd, would be desirable to confirm this. Pollution from sewage works and poor sanitation in nearby settlements probably present a greater risk to water quality.
The quarry does actively circulate water from the dewatering of pits, via the plant and various dams, recharging groundwater and then discharging back into the pits. The layout of the site contributes to this problem, with the plant and dams above the pits. Recirculation is, however, limited, as evaporation and salinity are only moderate, tempered by regional groundwater flow that keeps the groundwater and mine water fresh.
Recommendations
Recommendations for management of this and other mine sites include better planning of the site layout to prevent recirculation of mine water. In the event this is not possible, then lining of slurry dams to prevent infiltration could be a practical solution. Optimising explosives use, including storage and handling areas, may help reduce nitrate levels somewhat.
Recommendations for further study include analysing the groundwater for trace elements and microbiology, to better quantify the water quality. Stable isotope studies benefit from long-term precipitation data. Setting up long term monitoring sites that include such parameters could benefit many researchers working in the natural sciences, but especially the water sciences. Other tracers and parameters could also be used to help understand the surface water-groundwater interaction, such as tritium, useful to date young groundwater, or radon, useful to differentiate groundwater from surface water.
Mining companies are generally reluctant to allow research on their sites or use of their data. We are very grateful to PPC Mooiplaas and hope that this research encourages other mines to open up to research, as this would benefit the broader research community, being able to share experiences and data, and in turn benefit society and the environment.
Data availability
Complete raw data is available from the corresponding author, upon request.
References
APHA (2005) Standard methods for the examination of water and wastewater. 22nd edition. Amer Pub Health Assoc, Washington DC
Baloyi RS, Diamond RE (2019) Variable water quality of domestic well emphasizes the need for groundwater quality monitoring and protection: Stinkwater, Hammanskraal, Gauteng. Water SA 45:216–224. https://doi.org/10.4314/wsa.v45i2.08
Cook P (2020) Introduction to isotopes and environmental tracers as indicators of groundwater flow. The Groundwater Project, Guelph, Ontario, Canada http://www.gw-project.org/ ISBN: 978-1-7770541-8-2
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
CSAG Climate Systems Analysis Group (2022) https://cip.csag.uct.ac.za/
Dent MC, Lynch SD, Schulze RE (1989) Mapping mean annual and other rainfall statistics over southern Africa. Technical Report 109/1/89. Water Research Commission, Pretoria
Diamond RE (2022) Stable isotope hydrology. Groundw Proj Guelph Ont. https://doi.org/10.21083/978-1-77470-043-3
Dippenaar MA, Van Rooy JL, Diamond RE (2019) Engineering, hydrogeological and vadose zone hydrological aspects of Proterozoic dolomites (South Africa). J Afr Earth Sci 150:511–521. https://doi.org/10.1016/j.afrearsci.2018.07.024
Doveri M, Natali S, Franceschi L, Menichini M, Trifiro S, Giannecchini R (2021) Carbonate aquifer threatened by legacy mining: hydrodynamics, hydrochemistry and water isotopes integrated approach for spring water management. J Hydrol 593:125850. https://doi.org/10.1016/j.jhydrol.2020.125850
DWAF (1999) Johannesburg, 1:500,000 hydrogeological map. Dept of water affairs and forestry, Pretoria
Eary LE (1999) Geochemical and equilibrium trends in mine pit lakes. Appl Geochem 14:963–987
Eriksson PG, Altermann W, Hartzer FJ (2006) The Transvaal Supergroup and its precursors, pp 237–260. In: Johnson MR, Anhaeusser CR, Thomas RJ, The Geology of South Africa. Geological Society of South Africa, Johannesburg and Council for Geoscience, Pretoria
Gammons CH, Brown A, Poulson SR, Henderson TH (2013) Using stable isotopes (S, O) of sulfate to track local contamination of the Madison karst aquifer, Montana, from abandoned coal mine drainage. Appl Geochem 31:228–238. https://doi.org/10.1016/j.apgeochem.2013.01.008
Gao X, Wang Y, Ma T, Hu Q, Xing X, Yu Q (2011) Anthropogenic impact assessment of Niangziguan karst water. Water Manag 164:495–510. https://doi.org/10.1680/wama.1000070
Germishuys R, Diamond RE (2022) Nitrogen isotopes of Eichhornia crassipes (water hyacinth) confirm sewage as leading source of pollution in Hartbeespoort Reservoir. South Africa SA J Sci. https://doi.org/10.17159/sajs.2022/11098
Gleeson T, Befus KM, Jasechko S, Luijendijk E, Cardenas MB (2015) The global volume and distribution of modern groundwater. Nat Geosci. https://doi.org/10.1038/NGEO2590
Hobbs P, De Meillon N (2017) Hydrogeology of the sterkfontein cave system, cradle of humankind, South Africa. SA J Geol 120:403–420. https://doi.org/10.25131/gssajg.120.3.403
IAEA (2009) Laser spectroscopic analysis of water samples for stable hydrogen and oxygen isotopes. Training Course Series 35, International Atomic Energy Agency, Vienna, Austria
Jasechko S, Perrone D (2021) Global groundwater wells at risk of running dry. Science 372:418–421
Kåresdotter E, Destouni G, Ghajarnia N, Lammers RB, Kalantari Z (2022) Distinguishing direct human-driven effects on the global terrestrial water cycle. Earth’s Future. https://doi.org/10.1029/2022EF002848
Knüppe K (2011) The challenges facing sustainable and adaptive groundwater management in South Africa. Water SA 37:67–79
Kummu M, Ward PJ, De Moel H, Varis O (2010) Is physical water scarcity a new phenomenon? Global assessment of water shortage over the last two millennia. Environ Res Lett 5:034006. https://doi.org/10.1088/1748-9326/5/3/034006
Meyer R (2014) Hydrogeology of Groundwater Region 10: The Karst Belt. WRC report TT553/14. Water Research Commission, Pretoria
Nhemachena C, Nhamo L, Matchaya G, Nhemachena CR, Muchara B, Karuaihe ST, Mpandeli S (2020) Climate change impacts on water and agriculture sectors in Southern Africa: Threats and opportunities for sustainable development. Water 12:2673. https://doi.org/10.3390/w12102673
Nilsson L, Widerlund A (2017) Tracing nitrogen cycling in mining waters using stable nitrogen isotope analysis. Appl Geochem 84:41–51. https://doi.org/10.1016/j.apgeochem.2017.05.025
Nordstrom DK (2011) Hydrogeochemical processes governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to surface waters. Appl Geochem 26:1777–1791. https://doi.org/10.1016/japgeochem.2011.06.002
Oki T, Kanae S (2006) Global hydrological cycles and world water resources. Science 313:1068–1072
Page DC, Du Plessis PG (1986) Chert free metallurgical grade dolomite in the southern Transvaal the Mooiplaas and Glen Douglas Mines, In: Anhaeusser CR, Maske S, Mineral Deposits of Southern Africa, Geological Soc of South Africa, Johannesburg pp 829–835
Pietersen K (2006) Multiple criteria decision analysis (MCDA): a tool to support sustainable management of groundwater resources in South Africa. Water SA 32:119–128
Poeter E, Fan Y, Cherry JA, Wood W, Mackay D (2020) Groundwater in our water cycle-getting to know Earth’s most important fresh water source. Groundwa Proj Guelph Ont Canada. https://doi.org/10.21083/978-1-7770541-1-3
Rimayi C, Chimuka L, Gravell A, Fones GR, Mills GA (2019) Use of the Chemcatcher passive sampler and time-of-flight mass spectrometry to screen for emerging pollutants in rivers in Gauteng Province of South Africa. Environ Monit Assess 191:388. https://doi.org/10.1007/s10661-019-7515-z
SABS (2001) South African Standard SABS 241—Drinking Water. South African Bureau of Standards, Pretoria
SAWS (South African Weather Service) (2021) Regional weather and climate of South Africa: Gauteng. Unpublished report: WCS-CLS-REGIONAL_CLIMATE_GAUTENG.001
Schrader A, Winde F, Erasmus E (2014) Using impacts of deep-level mining to research karst hydrology—a Darcy-based approach to predict the future of dried-up dolomitic springs in the Far West Rand goldfield (South Africa). Part 1: a conceptual model of recharge and inter-compartmental flow. Environ Earth Sci 72:3549–3565. https://doi.org/10.1007/s12665-014-3263-0
Schrader A, Winde F (2015) Unearthing a hidden treasure: 60 years of karst research in the Far West Rand, South Africa. SA J Sci 111:2014–2144. https://doi.org/10.17159/sajs.2015/20140144
Simonovic SP (2002) World water dynamics: global modeling of water resources. J Environ Manag 66:249–267. https://doi.org/10.1006/jema.2002.0585
Sun J, Tang C, Wu P, Strosnider WH (2014) Hydrogen and oxygen isotopic composition of karst waters with and without acid mine drainage: impacts at a SW China coalfield. Sci Total Environ 487:123–129. https://doi.org/10.1016/j.scitotenv.2014.04.008
UNESCO (2022) The United Nations World Water Development Report 2022: Groundwater: Making the invisible visible. UNESCO, Paris
Van Wyk E, Van Tonder GJ, Vermeulen D (2011) Characteristics of local groundwater recharge cycles in South African semi-arid hard rock terrains - rainwater input. Water SA 37:147–154
Weather & Climate (2023a) Pretoria weather in February 2017. https://weatherandclimate.com/south-africa/gauteng/pretoria/february-2017
Weather & Climate (2023b) Pretoria weather in May 2017. https://weatherandclimate.com/south-africa/gauteng/pretoria/may-2017
Wikipedia (2022) https://en.wikipedia.org/wiki/gauteng
Winde F (2013) Challenges for sustainable water use in dolomitic mining regions of South Africa—a case study of uranium pollution part I: Sources and pathways. Phys Geogr 27:333–347. https://doi.org/10.2747/0272-3646.27.4.333
Zhu G, Wu X, Ge J, Lui F, Zhao W, Chu Wu (2020) Influence of mining activities on groundwater hydrochemistry and heavy metal migration using a self-organizing map (SOM). J Cleaner Prod 257:120664. https://doi.org/10.1016/j.jclepro.2020.120664
Acknowledgements
The authors thank Pretoria Portland Cement Mooiplaas Dolomite Quarry for access to the quarry for sampling and agreeing to release historical data, Aquatico Laboratories for assistance with sampling, the Water Research Commission for funding, and Grant Hall and Stefan Woodborne at the University of Pretoria stable isotope laboratory for analytical assistance.
Funding
Open access funding provided by University of Cape Town. Water Research Commission, South Africa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Diamond, R., van Staden, C. & Dippenaar, M. Tracing Mine Water Flows in a Dolomite Quarry, South Africa, Using Hydrochemistry and Stable Isotopes. Mine Water Environ (2024). https://doi.org/10.1007/s10230-024-00980-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10230-024-00980-8