Abstract
Mine drainage from the St Louis Tunnel (located at the Rico-Argentine Site) is circumneutral most of the year, with spring freshets increasing flow, decreasing pH and increasing metals concentrations. This study was designed to test the performance of a demonstration-scale horizontal wetlands passive treatment train, comprised of a settling basin, surface flow wetland, horizontal-flow anaerobic wetland, aeration channel, and rock drain, during two years of influent water chemistry at a constant 113 L/min (30 gpm) flow rate. Total Zn, Cd, and Mn effluent concentrations met project treatment goals (PTGs) 75, 96.9, and 100% of the time, respectively, and 93.9, 100, and 100% of the time for the dissolved metals. Most PTG exceedances occurred during the freshet events. Most Zn and Cd attenuation was attributed to sulfide precipitation in the anaerobic cell and capture/filtration of suspended ZnS particles in the anaerobic wetland and rock drain. Manganese was attenuated in the aerobic portion of the anaerobic cell (influent transition zone) as Mn oxides and carbonates. Oxidation of Mn occurred in the rock drain as biogenically formed Mn oxides adhered to the rock matrix. Carryover of dissolved sulfides from the anaerobic cell limited the rock drain’s Mn removal efficiency. Low temperatures did not significantly affect biological activity within the system; the effects of seasonal water quality were more important.
Zusammenfassung
Das Grubenwasser am Standort der stillgelegten Rico-Argentine-Mine ist die meiste Zeit des Jahres zirkumneutral, wobei das Frühjahrshochwasser den Durchfluss erhöht, den pH-Wert senkt und die Metallkonzentrationen erhöht. In dieser Studie sollte die Leistung einer passiven Behandlungskette in horizontalen Feuchtgebieten im Demonstrationsmaßstab getestet werden, und zwar über einen Zeitraum von zwei Jahren bei einer konstanten Durchflussmenge von 113 l/min. Das System bestand aus einem Absetzbecken, einem Feuchtgebiet mit Oberflächenströmung, einem anaeroben Feuchtgebiet mit horizontaler Strömung, einem Belüftungskanal und einer Steinrigole. Die Gesamtkonzentrationen von Zn, Cd und Mn im Ablauf entsprachen jeweils in 75, 96,9 bzw. 100% der Zeit den Projektbehandlungszielen (PTGs), die der gelösten Metalle in 93,9, 100 bzw. 100% der Zeit. Die meisten PTG-Überschreitungen traten während der Hochwasserereignisse auf. Der größte Teil der Zn- und Cd-Minderung wurde der Sulfidausfällung in der anaeroben Zelle und der Abscheidung/Filtration von suspendierten ZnS-Partikeln im anaeroben Feuchtgebiet und in der Steinrigole zugeschrieben. Mangan wurde im aeroben Teil der anaeroben Zelle (Zulaufübergangszone) in Form von Mn-Oxiden und -karbonaten gemindert. Die Oxidation von Mn fand in der Steinrigole statt, indem die biogen gebildeten Mn-Oxide an die Steinmatrix anhafteten. Die Verschleppung von gelösten Sulfiden aus der anaeroben Zelle schränkte die Mn-Abscheideeffizienz der Steinrigole ein. Niedrige Temperaturen hatten keinen signifikanten Einfluss auf die biologische Aktivität innerhalb des Systems; die Auswirkungen der saisonalen Wasserqualität waren wichtiger.
Resumen
El drenaje de la mina cerrada Rico-Argentina es circunneutral la mayor parte del año, con los deshielos de primavera aumentando el flujo, disminuyendo el pH y aumentando las concentraciones de metales. Este estudio fue diseñado para probar el rendimiento de un tren de tratamiento pasivo de humedales horizontales a escala de demostración, compuesto por una cuenca de sedimentación, un humedal de flujo superficial, un humedal anaeróbico de flujo horizontal, un canal de aireación y un drenaje de roca, durante dos años de química de entrada de agua a un caudal constante de 113 L/min (30 gpm). Las concentraciones totales de Zn, Cd y Mn en el efluente cumplieron los objetivos de tratamiento del proyecto (PTG) el 75, 96,9 y 100% del tiempo, respectivamente, y el 93,9, 100 y 100% del tiempo para los metales disueltos. La mayoría de los excedentes respecto de los PTG se produjeron durante los episodios de deshielo. La mayor parte de la atenuación de Zn y Cd se atribuyó a la precipitación de sulfuro en la celda anaeróbica y a la captura/filtración de partículas de ZnS en suspensión en el humedal anaeróbico y en el drenaje de roca. El manganeso se atenuó en la parte aeróbica de la célula anaerobia (zona de transición del afluente) como óxidos y carbonatos de Mn. La oxidación del Mn se produjo en el drenaje de roca como óxidos de Mn formados biogénicamente y adheridos a la matriz de la roca. El arrastre de sulfuros disueltos desde la célula anaeróbica limitó la eficiencia de eliminación de Mn del drenaje de roca. Las bajas temperaturas no afectaron significativamente a la actividad biológica dentro del sistema; los efectos de la calidad estacional del agua fueron más importantes.
抽象的
在一年的大部分时间里, 已闭坑的Rico-Argentine矿的矿山排放废水呈近中性, 但春季洪水流量上涨时, 废水pH值降低, 金属浓度增大。研究旨在检验试点规模的水平流湿地被动处理系统以稳定流量113升/分钟 (30加仑/分钟) 处理近中性废水两年的运行性能。系统由一个沉淀池、表面流湿地、水平流厌氧湿地、曝气槽和填石沟组成。出水的总锌、镉和锰浓度分别在75%、96.9%和100%的时间内满足项目处理目标 (PTG), 它们的溶解态金属分别在93.9%、100%和100%的时间内满足项目处理目标。超过项目处理目标 (PTG) 的事件多数发生在洪水阶段。锌和镉的衰减主要归因于厌氧池内硫化物沉淀和厌氧湿地与填石沟内悬浮ZnS颗粒的捕获/过滤。锰在厌氧池的好氧部分 (进水过渡区) 变为锰氧化和碳酸盐而减少。锰在填石沟形成生物成因锰氧化物, 附着于岩石基质。厌氧池遗留的溶解硫化物限制了填石沟的锰去除效率。低温对系统的生物活动无明显影响, 而水质的季节性影响却更为重要。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Project Background
The St Louis Tunnel (SLT) is an adit that drains several underground workings at the Rico-Argentine Mine site near Rico, Colorado (Lewis-Russ et al. 2022). The adit produces 1670–5320 L/min (440–1400 gpm) of circumneutral mine drainage that varies seasonally and discharges via a set of ponds into the nearby Dolores River. The SLT drainage contains elevated concentrations of Cd, Mn, and Zn that require treatment. This study focuses on evaluating the performance of a horizontal wetland treatment train (HWTT) that employs a horizontal sub-surface flow anaerobic wetland (HFW) component and an aerobic limestone rock drain to remove Zn, Cd, and Mn. The primary purpose of this study was to evaluate the seasonal performance of the HWTT over the range of influent water chemistry for two years to develop design criteria for a full-scale system.
This is the third of three related papers regarding the Rico site project. The first paper, Lewis-Russ et al. (2022), presents site history and background information, including characterization of the seasonal SLT flow and composition. The second paper, Dean et al. (2022), presents design and performance evaluation for a vertical wetland treatment train (VWTT). This third paper presents results, performance evaluation, and lessons learned for the HWTT. Studies of the two treatment systems were conducted over the same time period, using the same seasonally varying influent water, at a nominal flow rate of 113 L/min (30 gpm) to facilitate evaluation of the technologies that would be further tested at the site.
Site Background
Detailed information on hydrology and geochemistry of mine drainage from the Rico site is contained in Lewis-Russ et al. (2022). In general, water flowing from the SLT is circumneutral and contains elevated concentrations of Cd, Cu, Fe, Mn, and Zn. An annual spring freshet occurs following snowmelt, during which flows nearly double, water pH decreases by > 0.5 units, and concentrations of Cd, Mn, and Zn increase 2- to 4-fold.
The Rico site is located at an elevation of ≈ 2740 m (9000 ft), with associated severe winter conditions of cold temperatures and heavy snow. These conditions restrict site access and present potential avalanche conditions, which partly led to evaluate passive or semi-passive treatment of SLT water to minimize seasonal risks to personnel and equipment. The site-specific conditions also represent a challenge to seasonal performance of this technology and required pilot testing to evaluate effectiveness on a year-round basis.
Methods
Horizontal Flow System Design
The HWTT configuration is well suited to sites with relatively low elevation gradients and shallow groundwater tables, such as at this location. Such configurations have long been used for treatment of municipal wastewater and have been applied to treat mine water (Fitch and Schoenbacher 2009; US EPA 1993). However, year-round performance of a passive treatment system remains to be demonstrated for sites such as Rico, with a wide range of influent water chemistry and harsh winter conditions.
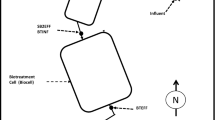
A layout of the HWTT is presented in Fig. 1. The system consists of the following membrane-lined unit operations, along with their primary purpose.
-
Settling basin #1 (SB1) (including 15 mg/L aluminum chlorohydrate coagulant dosing) was a gravity-fed, geomembrane-lined basin designed to settle suspended solids. It had a nominal hydraulic residence time (HRT) of 18 h. The performance objective for SB1 was to decrease total effluent Fe concentrations to less than 3.0 mg/L.
-
The surface flow wetland (SFW) was a gravity-fed, geomembrane-lined shallow pond filled with topsoil planted with nursery-grown water sedge (Carex aquatilis) and beaked sedge (Carex rostrata). This aerobic wetland was operated at 30 cm (1 ft) water depth and was designed to decrease influent elevated total Fe concentrations to < 0.5 mg/L.
-
The horizontal subsurface flow wetland (HFW) was a gravity-fed, geomembrane-lined pond filled with a 1.46 m (4.8 ft) deep layer of an organic matrix (rocks, wood chips, manure, and sulfur prills) designed to support sulfate-reducing bacteria (SRB), while maintaining an open porosity over a long service life. The HFW had two piping manifolds (influent and effluent) to promote uniform flow across the wetland. At a nominal HRT of 25 h, this anaerobic wetland was designed to produce effluent concentrations of less than 2.3 µg/L of dissolved Cd and 476 µg/L of dissolved Zn.
-
The aeration channel (AC) was a shallow, gravity-fed, geomembrane-lined, rock-filled channel with a nominal HRT of ≈ 68 min. It was designed to remove influent sulfide and provide aerated influent water to the rock drain. Three bubblers were installed to decrease sulfide concentrations to < 0.05 mg/L, reduce dissolved CO2, and increase dissolved oxygen (DO) levels to > 2.0 mg/L.
-
The rock drain (RD) was a gravity-fed, geomembrane-lined shallow pond filled with 1.04 m (3.4 ft) of 3.8–5.1 cm (1.5–2 in.) washed dolomitic limestone rock. The drain was operated with an HRT of 18 h. Design criteria were based on USBM criteria of 1 g Mn/m2/day (Hedin et al. 1994).
HWTT Flow
A constant flow of gravity-driven mine water was introduced to the HWTT through a flow diversion box (FDB) at a nominal rate of 113 L/min [30 U.S. liquid gallons per minute (gpm)]. Flow rates were adjusted manually with a gate valve in the FDB and measured with an electromagnetic meter; water levels within SB1, SFW, HFW, and RD were adjusted with an inline water level control structure (Agridrain™). Water levels within these system components were managed to optimize the system performance during the study.
Mine Water Sampling and Analysis
Three water quality sondes were deployed at permanent locations at the SB1 (SB1EFF), HFW (AC1INF), and RD (RDEFF) (Fig. 1). These sondes (YSI EXO1, and EXO2) were selected for their adaptations to long-term deployment in aquatic environments. They were equipped with sensors to measure pH, ORP, DO, temperature, conductivity, turbidity, and water depth. These sondes were calibrated monthly and periodically cleaned of accumulated biofilm.
Water samples were collected monthly (semi-monthly in some cases) from the inlets and outlets of each treatment unit. These samples were analyzed for metals and other water quality parameters by a commercial laboratory using standard U.S. Environmental Protection Agency (US EPA)-approved methods identified in Lewis-Russ et al. (2022). Experience of other wetland researchers has shown that it is important to analyze total (unfiltered) and dissolved (0.45 mm filtered before sample preservation and analysis) concentrations of metals and the term “dissolved metals” is interpreted as 0.45 mm filtered samples throughout this publication. Another term that will be used in the text is suspended particulate metals, which is total metals minus dissolved metals. Additionally, water samples were collected from monitoring ports along three transects within the HFW, and water quality parameters (temperature, conductivity, DO, pH, ORP, and turbidity) were measured using calibrated field instruments.
Many metal analyses for treated water were below reporting limits (RL) for the analytical methods used. For purposes of plotting and removal efficiency calculations, values of ½ RL were used where data were less than the reporting limit.
Rock Drain Investigation
Scrapings of black precipitates and staining on the rock media were collected near the inlet, middle, and outlet of the rock drain and submitted for chemical and mineralogical analysis, including strong acid extractable metals (EPA3050B, with EPA 6010), sequential extraction (Tessier et al. 1979, with EPA 6010), and scanning electron microscopy energy dispersive X-ray spectroscopy (SEM–EDS).
Remote Monitoring System
The sondes deployed in the HWTT were connected to a data logger and telemetry system that was accessible remotely, providing real-time data to authorized personnel for system management, including: pH, specific conductivity, ORP, DO, turbidity, and temperature (Dean et al. 2022). The monitoring system also included a weather station, sulfide monitors for safety evaluation, and a site camera. Real-time data were available to authorized site personnel, but system modifications were performed manually at the site.
Results
Mine drainage from the SLT was fed continually at ≈ 113 L/min (30 gpm) to provide the entire range of seasonal water quality over a two-year period (2015–2016). During that time, water quality parameters and metal concentrations were measured at locations throughout the treatment system (Fig. 1).
During the freshet season of 2015, it was determined that the monitoring well point for evaluating the HFW was faulty and not representative of the treated water from the HFW. The monitoring point was switched to a new location at the discharge of the HFW-treated water manifold to the aeration channel (subsequently termed AC1INF). An analysis of the sampling location change, included in the supplemental information, shows that metal concentrations in the HFW effluent are best represented by the AC1 effluent data (AC1EFF) up until 9/10/2015 combined with AC1INF data from 10/12/2015 forward to provide a continuous record over the study (more detail in supplemental information). Water parameters heavily affected by the air sparging in the aeration channel include sulfide, oxygen, and CO2 concentrations, pH, and ORP measurements. The data from the original sampling location (well HSSWMP11) and the replacement sampling point (AC1INF) are the continuous data best suited to HFW evaluation and will be referred to as HFWEFF.
Seasonal Changes in Water Chemistry
The spring freshet at the Rico site brought several changes to the SLT mine water chemistry, including increased metal concentrations and decreased pH and alkalinity concentrations (Dean et al. 2022; Lewis-Russ et al. 2022), which affected the performance of the HWTT. Time-series data for treatment water pH throughout the HWTT are contained in Fig. 2. Values of pH in the influent were depressed during both (2015 and 2016) freshets and those effects were noted throughout all components of the HWTT. The 2015 freshet was different in that the pH values at the HFWEFF were lower than the influent pH, which is attributable to oxidation of elemental sulfur (prills) that was added to the HFW media to assist in low temperature operations (Marsland et al. 2010). Figure 3 contains time-series pH and sulfide concentrations for HFW effluent (HFWEFF) and Fig. 4 contains sulfide at HFWEFF and dissolved Zn flowing into the HFW (HFWINF). Sampling for the freshet events occurred when the SLT flow initially increased and the pH (based on in-place sondes) decreased, according to the conceptual site model (Lewis-Russ et al. 2022). The 2016 freshet had anomalously low flow; a sampling event was conducted at the influent/effluent locations on May 26, 2016, out of concern that the 2016 freshet had begun. A full sampling of the system was performed on June 6, 2016; however, peak metals concentrations for some system components were missed. This illustrates the temporal variability and importance of understanding the conceptual site model and when to collect samples to characterize freshet seasons. The HFWEFF sulfide concentrations were very high during system startup, from 1 to 8 mg/L during non-freshet periods, and decreased abruptly to < 0.05 mg/L during both freshet periods. The effects of the freshet on sulfide concentrations lasted one month following the 2015 freshet. The pH and/or Zn concentrations affected sulfide production in the HFW (Figs. 3, 4). The EC:50 (Zn concentration reported to cause a 50% decrease in sulfate reduction in mixed SRB cultures, Utgikar et al. 2001) and lower pH range for optimal SRB activity are both plotted for reference.
Changes in pH and ORP distribution during the spring freshet were apparent spatially within the HFW, as measured at 11 locations across transects at the inlet, middle, and outlet positions of the HFW (Fig. 5). The 2015 results of pH and ORP measurements (Figs. 6 and 7, respectively) illustrate the seasonal dynamics of these parameters within the HFW. Figure 6 shows that ambient pH during the 2015 freshet decreased by 0.2–0.3 units as water travelled across the entire HFW, reaching a minimum of 5.9 at one individual location during the 2015 spring freshet. Water pH values drop consistently along the flow path, suggesting a form of acidity in the wetland media in addition to acidity from the influent water (sulfur prills). The narrow transition zone (inlet end) of the HFW stayed aerobic the entire year, but ORP decreased rapidly as water travelled across the wetland during the non-freshet periods (Fig. 7). During the spring freshet, however, the aerobic portion of this transition zone extended across the entire length of the HFW. The increased ORP and decreased pH in the middle and outlet end of the HFW during the 2015 freshet were sub-optimal for SRB activity as reflected in decreased levels of sulfide during and after freshet (Fig. 3). This is consistent with the optimal pH and ORP requirements of most known freshwater SRBs (Hartzell and Reed 2006; Widdel 1988; Widdel and Bak 1992).
Metal Removal in the Horizontal Wetland System
Time series plots of As, Cd, Cu, Fe, Mn, Ni, Pb, and Zn concentrations for each component of the HWTT (influent, SBEFF, SFWEFF, HFWEFF, ACEFF, and RDEFF) are contained in supplemental Figs. S-1–S-8. Site-specific effluent criteria for the Rico site are not yet available for performance evaluation; therefore, a 2008 water quality assessment (WQA, Colorado Dept. of Public Health and Environment (CDPHE 2008)) for a potential lime treatment plant was used to develop project treatment goals (PTGs) for this research project (Table 1). The WQA contains effluent criteria that are protective of the most limiting surface water beneficial use, including toxicity to aquatic species, without regard to treatment technology or process. Although the WQA values are for dissolved metals concentrations (0.45 µm filtered), CDPHE generally requires potentially dissolved metals analyses (acid digestible metals, unfiltered) until it can be demonstrated that dissolved analyses correlate with potentially dissolved analyses (CDPHE 2017). For this study, we had very few potentially dissolvable metal data, so total metals (unfiltered) analyses were used as a conservative comparison to system performance (PTGs). The PTGs are included in Figs. S-1–S-8 to assist in performance evaluation. The frequency of PTG exceedances and removal efficiency for metals and As are reported in Table 2.
The fate and transport of metals in the HWTT were initially affected by whether they were in dissolved or particulate form. Iron oxyhydroxide is the dominant suspended solid in the mine drainage influent to the HWTT and was largely removed by settling (in the SB and, to a lesser extent, the SFW). Pb, As, and to some extent Cu were adsorbed/coprecipitated with Fe oxyhydroxides and removed primarily in the SB and SFW. Table 2 illustrates that As, Cu, Fe, Ni, and Pb generally met PTGs in the HWTT effluent (following system startup period) and so are not discussed further. Cd, Mn, and Zn were predominantly dissolved in the influent, were not attenuated appreciably in the SB and SFW, and so are the key metals of interest in this paper.
Zinc was present in highest concentrations during both freshet and non-freshet conditions, with freshet Zn concentrations almost 100 times those of Cd. For the post-startup period, total Zn concentrations in HWTT effluent (RDEFF) were below the PTGs 75% of the time (Figs. 8, S-8, Table 2), while dissolved Zn met PTGs 93.8% of the time. The average monthly removal efficiency for total Zn was 91.8% (Table 2) with highest removal efficiencies in the non-freshet periods and removal efficiencies as low as 60% during freshet periods when total concentrations were highest. Most of the total Zn removal occurred in the HFW (Figs. 8, S-8) and most of the total Zn in the HFW effluent was in the particulate form (Fig. 9). Analysis of the particulate Zn in a companion study (this issue) indicated that the suspended particles were ZnS (Dean et al. 2022). Nanophase ZnS particles can have significant mobility in saturated media and are common in natural systems (Deonarine et al. 2011) and other wetland and passive treatment studies (Gammons et al. 2000; Jarvis et al. 2015). Total Zn concentrations exceeded PTGs at times in the HFWEFF; however, the RD was effective at capturing most of the suspended material before system discharge (RDEFF). Post startup, total Zn only exceeded PTGs during the freshet periods and two samples during late 2016 (Fig. 9). In the more oxic environment of the RD, the dissolved Zn concentrations in the RDEFF were higher than upstream in the HFWEFF and dissolved Zn for the RDEFF (HWTT effluent) tended to increase over time.
Cadmium behaved similarly to Zn; however, much lower concentrations in the HWTT influent resulted in more frequent achievement of Cd PTGs, despite lower PTGs for Cd. Total Cd concentrations in the HWTT effluent (RDEFF) were below PTGs except for once during the system startup period and once during the 2015 freshet (Figs. 10, S-2, Table 2). Frequency of meeting PTGs after the startup period was 96.9% for total Cd and 100% for dissolved Cd (Table 2). The average total Cd concentration in the HWTT effluent after the startup period was 0.53 µg/L compared to the reporting limit of 0.5 µg/L (value of ½ the reporting limit used for data < RL). Most Cd mass removal was in the HFW (Figs. 10 and S-2) and most of the Cd in the effluent of the HFW was in the particulate form as CdS or Cd substituted into the ZnS structure (Fig. 11). The molar ratio of Cd:Zn in the HWTT influent was 0.3% averaged over the project and substitution of Cd in ZnS is the most probable Cd removal mechanism. Some of the total Cd was also removed in the RD, with most being settled as particulate matter (Figs. 10, S-2).
Total Mn concentrations were attenuated by the HWTT at an unexpectedly high efficiency (Figs. 12, S-5). Total and dissolved Mn met PTGs 100% of the time following the system startup period (Table 2). Post startup, attenuation of Mn was high in both the HFW and the RD components of the HWTT system (Fig. 12). The upgradient HFW removed most of the Mn, except during startup and the 2015 freshet. The Mn attenuation was negatively affected one month into the 2015 freshet and dissolved Mn from the HFWEFF increased above the lower PTG. The RD compensated for the HFW’s reduced attenuation during the 2015 freshet and prevented exceedance of PTGs at system effluent (RDEFF). As the study progressed, the ability of the HFW and RD to attenuate Mn improved. After Jan. 6, 2016, the HWTT effluent (RDEFF) averaged 14.3 µg/L for total Mn (calculated using ½ the reporting limit of 10 µg/L for < RL data) and an average removal efficiency of 99.4%. Unlike Zn and Cd concentrations that were dominated by particulate metals in the HFWEFF, Mn was almost completely dissolved, illustrating a different attenuation mechanism (Fig. 13).
Analysis of Precipitates in the RD and HFW
A chemical and mineralogical analysis of the precipitates and black deposits on surfaces of limestone rock media showed that greater attenuation of both Mn and Zn occurred in the upgradient position of the RD (Table 3). Visual observations of staining indicated that most Mn precipitation occurred in the upper portion of rock media (sample from 45 cm depth), with limited staining from 80 cm to the full depth (1 m) of the limestone media (Fig. 14).
Black deposits in rock drain (possibly pyrolusite). Photo illustrates significant black coatings on rock matrix in the upper saturated zone (45 cm) compared to rock near the bottom of the rock drain (80 cm) at the same location. The handwritten “water surface” in the photograph refers to the water surface in the dewatered sampling trench and not the operational water surface for the wetland cell
Results from sequential extractions based on Tessier et al (1979) indicate that Mn was recovered primarily in the hydroxylamine extraction step, which is indicative of Mn oxides (Table 4). Analysis of RD particles and aggregates by SEM–EDX confirmed that the Mn oxides were primarily biogenic forms that occurred as tubular sheaths that formed as pseudomorphs around microbial colonies (Fig. 15). Microbial formation of Mn oxides have been reported in wetlands and passive treatment systems and natural systems (Hallberg and Johnson 2005; Luan et al. 2012). Particles of ZnS were also noted in the RD, resulting from sedimentation of particles transported from the HFW (Fig. 16).
Biogenic forms of Mn precipitates found adhering to the rock drain matrix. These are believed to be Mn Oxides. The limestone matrix rock contributes to the EDS spectral analysis computation. Elemental composition expressed as weight percent (wt%) and atomic percentage (at%). The “K” following the element symbol signifies the energy peak used for quantification
Precipitates of Mn in the HFW were more difficult to identify. Manganese carbonates were common in the effluent end of the HFW (Fig. 17). The SEM–EDS analysis of the ≈ 2 mm red box in the micrograph had an average composition of Mn3Zn1Fe0.75Ca0.25(CO3)5. It cannot be determined, without additional mineralogic information, if this was a single phase, solid solution of carbonates, or contributions from several small particles excited by the electron beam.
Seasonal Water Temperatures in the HWTT
Cold winter ambient air temperatures and the ability to maintain active SRB populations in the HWTT was a concern prior to the study. Water temperature was continually recorded by thermistors located throughout the HWTT and are expressed as average monthly temperatures in Fig. 18. Influent mine drainage was consistently warm over the course of the study (18.3–19.3 °C) and reflects the hydrothermal source of mine water emerging from underground. Water temperature measurements ranged from 5 to 19 °C across the system (Fig. 18), despite winter ambient air temperatures decreasing below −20 °C. Temperatures in the HWTT effluent (RDEFF) ranged from 5 to 17 °C.
Managing the HWTT with Water Data
The sondes installed within the HWTT enabled continuous monitoring of water quality parameters (temperature, conductivity, pH, ORP, turbidity, and DO) at their locations (Fig. 1). Information from the sensors was remotely accessible and was used to manage the HWTT. For instance, water levels in the HFW were controlled in response to effluent ORP and sulfide concentrations. Water levels were manually raised when ORP was elevated and effluent sulfide concentrations were low, to increase HRT. Alternatively, water levels were lowered when effluent sulfide concentrations were excessive. Most changes were made during system startup and non-freshet sulfide concentrations in the HFW effluent ranged between 1 and 8 mg/L with very few additional adjustments required. Timing of performance monitoring was eventually based on minor changes in influent pH instead of minor changes in water flow.
Discussion
The HWTT system treated the mine water to very low concentrations of Cd, Mn, and Zn over the 2 year period at the Rico mine site (Table 2). The startup period of the HWTT was about two months long, when microbial populations were being established and dissolved organic carbon was very high (based on visible brown coloration). High DOC initially passed down to the RD, which had the lowest ORP (-400 mV) at one point in the system initiation. Better treatment performance was noted for all analytes after the startup period. The behavior of Zn and Cd within the HWTT was similar; however, meeting Zn PTGs was more challenging. Cd concentrations averaged 0.5% and 0.6% (wt:wt) of the influent concentrations of Zn during freshet and non-freshet periods (respectively) and Zn attenuation constituted most of the sulfides precipitated in the media; therefore, this discussion will primarily address Zn. Mn only failed to meet the lower PTG for the study during the startup period and met PTGs 100% of the time during both freshet and non-freshet periods (both total and dissolved concentrations).
Zinc Chemistry
Attenuation of Zn in the HWTT was due primarily to sulfide precipitation, as reported by others (Dean et al. 2022; Gammons and Frandsen 2001; Gusek and Figueroa 2009). Seasonal changes during freshets (pH, sulfate and metals concentrations, Lewis-Russ et al. 2022) had important effects on SRBs, the consortia of microorganisms that support SRBs, SRB activity, and the effectiveness of Zn treatment in the HWTT. In the HFW, sulfide generation adequately removed Zn until the pH decreased to a level of 6.2 (Fig. 3). This is near the lower limit commonly reported for SRBs (Al-Zuhair et al. 2008; Bijmans et al. 2011; Dean et al. 2022; Widdel 1988; Widdel and Bak 1992; Zhang and Wang 2016). Specific metals toxicity within the HFW may also have inhibited SRB activity, as demonstrated by dissolved Zn entering the HFW at 13.7 mg/L (freshet of 2015) compared to the Zn EC:50 value (concentration where SRB activity is reduced by 50%) of 14 mg/L, determined for mixed SRB cultures in the laboratory (Utgikar et al. 2001) (Fig. 4). Precipitation of ZnS in the HFW can reduce metal toxicity, but the ZnS particles themselves can encapsulate bacteria and reduce sulfide generation rates (Utgikar et al. 2002). The pH and ORP in the HFW became more acidic and oxic in the middle and effluent portions of the cell during the freshet periods (Figs. 6, 7, respectively). It is likely that lower sulfide concentrations discharged from the HFW during freshet periods occur due to the cumulative effects of pH, metals toxicity, and precipitation of sulfides by Zn and other divalent metals. Since sulfide concentrations in HFWEFF were reduced to the detection limit of 0.05 mg/L during the freshet, it is important to know if SRB activity stopped during the freshet and the month following or if it was only inhibited.
A mass balance for the attenuation of Zn in the HFW is contained in Fig. 19. The Zn concentration units and attenuation and sulfide concentrations have been changed to µmol/L since sulfide precipitation is the dominant attenuating mechanism for Zn and Cd. The Zn in HFW influent makes up > 99% of the mass of sulfides precipitated, so performing the mass balance on Zn alone is adequate. The dissolved HFWINF Zn concentrations minus the dissolved HFWEFF Zn roughly equals the ZnS precipitation within the HFW, and the residual sulfide concentration in the HFW effluent is the reserve precipitation capacity not utilized. The HFW ZnS precipitation plus the residual sulfide is the total precipitation capacity (TPC) for the HFW at each sampling period. These two parameters and the HFWINF dissolved Zn and HFWEFF total and dissolved Zn have been plotted in Fig. 19. For the three months prior to the 2015 and 2016 freshet seasons, the TPC of the HFW averaged 190 and 114 µmol/L, respectively (eliminating the anomalously low sulfide value at 4/23/2015). The TPCs were exceeded by peak freshet influent dissolved Zn concentrations of 199 and 165 µmol/L (13,000 and 10,800 µg/L) for 2015 and 2016, respectively. These were the only times that dissolved Zn concentrations exceeded the lower PTG in the HFWEFF. Dissolved Zn concentrations in the HFWEFF were less than calculated by stoichiometry; this is likely due to hydrodynamic dispersion and utilization of the sulfide produced in less permeable portions of the HFW during the previous few months before the freshet peak Zn concentrations. It is important to note that the TPC decreased during, and for a month after, the freshet; this could reflect inhibition of the SRB activity by a number of factors, including: (1) influent dissolved Zn concentrations nearing the Zn EC:50 value of 14,000 µg/L (217 µmol/L) (Utgikar et al. 2001); (2) encapsulation of SRB colonies by ZnS particulates (Utgikar et al 2002); and (3) lower pH values (6.2) in the HFWEFF. The total concentration of Zn in the HFWEFF peaks (Fig. 19) of 54.5 and 87.2 µmol/L (3560 and 5700 µg/L) during 2015 and 2016 freshets account for 27 and 51% of the HFWINF dissolved Zn concentrations (respectively). The total precipitation capacity (conversion of ZnS and residual sulfide) appears to remain significant through the freshet. The lack of particulate ZnS captured in the HFW matrix appears to be as important to system effectiveness as the diminished conversion of dissolved Zn to ZnS.
The rock drain was effective in attenuating Zn post-HFW and kept the total Zn below PTGs during most non-freshet conditions (Fig. 9). Over the study, 90% of the total Zn concentrations at the HFWEFF consisted of Zn particulates (Fig. 9), which were captured as sediment within the limestone rock media in the RD (Fig. 16) and accumulated over time. However, the RD became more oxidizing over time, which resulted in oxidation of some of the captured ZnS sediment (Fig. 9). During the post-freshet months of 2016, the dissolved portion of the total Zn increased and the RDEFF exceeded PTGs slightly on only two sampling dates. Adsorption of dissolved Zn may occur on Mn oxides (Drever 1988); this is addressed in the chemical extraction discussion and Tables 3 and 4. Post-HFW removal of ZnS particles from the treatment waters will be an opportunity of future treatment system designs.
Attenuation of dissolved Zn involves precipitation of ZnS (and, to a lesser extent, Zn carbonates) followed by capture of ZnS suspended particles in the HFW and RD (Table 4). Nanophase or colloidal particles of ZnS are common in natural waters (Deonarine et al. 2011) and wetland systems (Dean et al. 2022; Gammons et al. 2000; Jarvis et al. 2015) and are somewhat mobile in saturated media. Naturally-occurring organic matter can adsorb to ZnS particles, limit particle-to-particle interactions and aggregate formation, and favor suspension of nanophase ZnS particles (Deonarine et al. 2011). Table 5 shows saturation indices for HWTT influent waters with respect to Zn, Mn, and Cd minerals using Geochemist’s Workbench (Bethke et al. 2020). A saturation index (SI) is the log10 of the ratio of the ion activity product (IAP), calculated using collected water chemistry data, to the solubility product constant K calculated using actual ion activities and a reference mineral standard at equilibrium (SI = log10 (IAP/K). Dissolved metals concentrations (0.45 mm filtered) were used to represent true dissolved metals concentrations. When the SI = 0, the solutions are in equilibrium with the mineral. When the SI is > 0, there is the potential for that minerals to precipitate. When the SI < 0, there is a tendency for the mineral to dissolve, if it is present in the system. Mine water is saturated (SI = 0) with respect to smithsonite when it exits the SLT and becomes slightly supersaturated (SI > 0) when treatment waters degas CO2 and pH rises in the SB1 and SFW. Zinc precipitated as Mn carbonates onto the rock media at the effluent end of the HFW (Fig. 17), as noted by others in natural systems (Hansel et al. 2002; LaForce et al. 2002). Smithsonite becomes undersaturated when treatment waters exit the HFW (and in the remainder of the system) because sphalerite (ZnS) precipitation in the HFW controls dissolved Zn concentrations. The SIs reflect supersaturation with respect to sphalerite and other sulfides there due to a combination of effects, including passage of nanoparticles containing Zn, Cd, Fe, and Ni that passed through the 0.45 mm sampling filters, slow kinetics of ZnS precipitation, and the effects of organics on ZnS precipitation and nanophase ZnS particle stabilization and suspension. Observation of individual ZnS particles in the HFW by SEM–EDS was not possible due to the generally small particle size. Some larger ZnS particles were identified by SEM–EDS as settled particles in coatings on RD limestone rock (Fig. 16). Total chemical extraction analysis of coatings and precipitates in the RD noted higher concentrations of Zn and Mn in the upstream end of the RD than in the downstream sample (Table 3). Sequential extraction analysis of rock coatings indicated that the hydroxyl amine extracts (indicative of Mn oxide extraction) also had greater concentrations of Zn and Mn in the upstream end of the RD, and that Zn was predominantly associated with the Mn oxide fraction. These data support that Zn is attenuated by ZnS precipitation and particle filtration in the HFW. Particulate ZnS in the RD appears to be attenuated by settling on the rock matrix, but the more oxidizing environment of the RD results in some Zn dissolution. Dissolved Zn concentrations appear to be offset to a large extent by adsorption to Mn oxides (Drever 1988; Förstner and Wittmann 2012) actively precipitated in the more oxidizing environment of the RD (explained below).
Manganese Chemistry
Manganese was effectively attenuated in both the horizontal flow wetland (HFW) and RD components. A time series plots of HFW influent Mn concentrations, Mn attenuation calculated at the HFW effluent (HFWEFF) and RDEFF, and sulfide concentrations at the AC1EFF are provided in Fig. 20. The AC1EFF location was used because it best represents the carryover of sulfide to the RD and the change from a reducing environment (HFW) to a more oxidizing environment in the RD. Attenuation of Mn in the HFW wetland (HFWEFF) was unexpected and is important because it is upgradient of the RD and attenuated most of the bulk Mn mass in the HWTT. Attenuation reached about 60% efficiency quickly after system start up, though the freshet of 2015 inhibited Mn attenuation in the HFW; a month or more was required to recover from the effects of the low pH and elevated metals on microbial activity. Manganese-oxidizing bacteria have an optimal pH range of 6.5–8 (Li et al. 2019), which is even narrower than that of SRBs. The spike in Mn attenuation efficiency at the beginning of the 2015 freshet reflects some lag time between the arrival of freshet effects at the influent and HFWEFF locations. Attenuation of Mn in the HFW increased in efficiency following the 2015 freshet and was not inhibited during the 2016 freshet as the system matured. Factors affecting maturation may include acclimation of Mn-oxidizing bacteria or the autocatalytic nature of Mn oxidation, which improves Mn removal over time. Attenuation of Mn in the RD is important as it reduced Mn concentrations to low values before final HWTT discharge at the RDEFF. Attenuation of Mn in the RD (area between the HFWEFF and RDEFF lines, Fig. 20) reached high levels shortly after system startup but declined prior to the 2015 freshet. As the freshet affected sulfide production, the sulfide concentrations that report to the RD decreased, and Mn attenuation in the RD increased. Thus, Mn attenuation in the RD is antithetic to the amount of sulfide generated and carried over from the HFW. A malfunction of the aeration equipment in AC1 on 10/12/2015 could have resulted in high carry over and higher sulfide concentrations at AC1EFF, which drove Mn attenuation in the RD to zero. Total Mn concentrations in the HWTT effluent (RDEFF) averaged 14.3 µg/L from 1/6/206 until the end of the study (using ½ the reporting limit of 10 µg/L for < RL data) and met lower PTGs year-round after startup (Table 2). This is a much lower level than previously reported for these systems (Rose et al. 2003).
A sequential leach analysis (Table 4) indicated that Mn was predominantly attenuated in the RD as an oxide. There were minor amounts of Mn extracted from carbonates and sulfide in that analysis. Domination by Mn-oxides is consistent with black deposits of the surface of limestone rock (Fig. 14), presumably pyrolusite. Others have reported similar depositions of pyrolusite in limestone-based rock drains and designs are based on the same treatment process (Rose et al 2003; Skousen et al 2017).
The initial sizing criteria for the RD followed well-established design criteria (Hedin et al 1994; Skousen et al 2017). However, treatment performance improved over time and the Mn in the rock drain effluent (HFWEFF) decreased to levels well below the discharge Mn goals used to develop these criteria. Removal of Mn in the HFW may account for the better-than-expected performance.
The only MnS phase data available (alabandite) was undersaturated throughout the system (Table 5), although other phases may occur. Precipitation of carbonates occurred to some extent (Fig. 17), although these could have been microsites of reducing activity and alkalinity generation not in equilibrium with the HFWEFF (Table 5). The saturation index for rhodochrosite drops across the HFW, which indicates that another, less soluble mineral was precipitating in the HFW. Since the sulfide produced in the HFW decreased over time, the transition zone may better support Mn oxidation and precipitation of oxides in the upgradient portion of the HFW. Rhodochrosite saturation indices for the HFWEFF show increasing undersaturation over time, similar to the increasing HFW Mn attenuation rates for the RDEFF over time.
Attenuation of Mn in the RD involves precipitation of biogenic Mn oxides that tend to adhere to the rock matrix of the RD. These sheaths or coatings of Mn oxides form pseudomorphs after the colonies of the microorganisms and have been noted by others (Hallberg and Johnson 2005; Luan et al. 2012). Sequential extraction of Mn oxides shows limited Zn in the Mn oxides (33.9 molar ratio of Mn:Zn, Table 4) and reflects the higher dissolved Mn:Zn values that carryover from HFW to the RD.
Influence of Water Temperature on Biological Activity
Water temperature at the HWTT effluent (RDEFF) is plotted along with sulfide concentrations in the HFWEFF and Zn removal efficiency in Fig. 21. Low temperatures do not correspond with low sulfide concentrations or system effectiveness and some of the low sulfide periods correspond to increasing water temperature periods. Sulfide lows correspond to freshet periods in spring when increasing temperatures, snow melt, increasing mine drainage, lower pH, and higher metals concentrations occurred in the HWTT influent (Lewis-Russ et al. 2022). These seasonal changes in the influent water quality overwhelmed any changes resulting from seasonal water temperatures within the HWTT. Other studies have shown that Mn removal in wetland or passive treatment systems may be reduced during cold seasons (Gammons and Frandsen 2001) or relatively insensitive in limestone rock drain systems above 5 °C (Rose et al. 2003). Removal efficiency of Mn measured at the RDEFF in this system was > 97% throughout 2016, while water temperature in the RDEFF ranged from 6 to 16 °C (Fig. S9), indicating low temperature sensitivity over the broad range tested.
Conclusions
The horizontal wetlands treatment train pilot-scale system was effective in controlling metals from a constant flow rate of mine water discharge at the Rico site. The system met project treatment goals at a high frequency, with total Zn being the most difficult to manage.
Seasonal water chemistry was the greatest challenge, with spring freshets producing lower pH and higher metals concentrations in the system influent. Inhibition of SRB activity resulted from a combination of effects from lower pH and higher Zn concentrations.
The anaerobic horizontal flow wetland (HFW) contributed the greatest attenuation of both Zn and Mn in the system. Zn was precipitated as ZnS and limited carbonates. During the freshet season, carryover of up to 50% of the influent dissolved Zn through the HFW was in the form of suspended particulate Zn (total–dissolved Zn), which was captured for the most part in the rock drain. Dissolved Mn was attenuated in the HFW as carbonates and possibly Mn oxides in the oxic transition zone.
The rock drain contributed to Mn and Zn attenuation in the system. Formation of biogenic Mn oxides attenuated total and dissolved Mn and adsorbed Zn released through oxidation of ZnS particulates.
There were no quantifiable effects of seasonal water temperature on the system’s effectiveness. The 18–19 °C year-round mine water inflow to the system kept the lowest discharge temperatures to greater than 5 °C. Seasonal fluctuations of influent mine water chemistry tended to overwhelm any temperature effects.
Inclusion of elemental sulfur in the HFW media mix to increase system effectiveness at low temperatures was not beneficial, but rather had a negative effect on sulfide production due to lowering of HFW pH on oxidation.
References
Al-Zuhair S, El-Naas MH, Al-Hassani H (2008) Sulfate inhibition effect on sulfate reducing bacteria. J Biochem Technol 1(2):39–44
Bethke CM, Ferrell B, Sherifi M (2020) Geochemist’s workbench: essentials guide (Release 14). Aqueous Solutions, LLC, Champaign (April 18, 2020)
Bijmans MFM, Buisman CJN, Meulepas RJW, Lens PNL (2011) Sulfate reduction for inorganic waste and process water treatment, chapter 6.34. In: Moo-Young M (ed) Comprehensive biotechnology, 2nd edn. Academic Press, Cambridge, pp 435–446. https://doi.org/10.1016/B978-0-08-088504-9.00471-2
CDPHE (Colorado Dept of Public Health and Environment) (2008) Water quality assessment for the St. Louis Tunnel discharge to the Dolores River. Provided by Eric T. Oppelt, Water Quality Control Div., Permits Section, with cover letter on Oct. 29, 2008
CDPHE (2017) Water Quality Control Commission. regulation no. 31—the basic standards and methodologies for surface water, 5 CCR 1002-31. https://www.colorado.gov/pacific/sites/default/files/31_2017-03.pdf. Accessed 28 Dec 2020
Dean DM, Fricke JR, Riese AC, Moore TJ, Brown AR (2022) Passive treatment of circumneutral mine drainage from the St. Louis mine tunnel, Rico, CO: part 2—vertical biotreatment train pilot study. Mine Water Environ 41 (in this issue)
Deonarine A, Lau BLT, Aiken GR, Ryan JN, Hsu-Kim H (2011) Effects of humic substances on precipitation and aggregation of zinc sulfide nanoparticles. Enviro Sci Technol 45:3217–3223
Drever JI (1988) The geochemistry of natural waters, 2nd edn. Prentice Hall, Hoboken
Fitch M, Schoenbacher J (2009) Performance of a full-scale horizontal-flow wetland for zinc. In: Barnhisel RI (ed) Proceedings of national meeting of the American society of mining and reclamation, pp 433–450. https://doi.org/10.21000/JASMR09010433
Förstner U, Wittmann GTW (2012) Metal pollution in the aquatic environment. Springer, Berlin
Gammons CH, Frandsen AK (2001) Fate and transport of metals in H2S-rich waters at a treatment wetland. Geochem Trans. https://doi.org/10.1186/1467-4866-2-1
Gammons CH, Mulholland TP, Frandsen AK (2000) Comparison of filtered vs. unfiltered metal concentrations in treatment wetlands. Mine Water Environ 19(2):111–123
Gusek JJ, Figueroa L (2009) Mitigation of metal mining influenced water: management technologies for metal mining influenced water, vol 2. Society of Mining, Metallurgy, and Exploration, Inc., Littleton, CO
Hallberg KB, Johnson DB (2005) Biological manganese removal from acid mine drainage in constructed wetland and prototype bioreactors. Sci Total Environ 338:115–124
Hansel M, LaForce MJ, Sutton S, Fendorf S (2002) Ecosystem dynamics of zinc and manganese within a mine-waste impacted wetland. In: Hellman R, Wood SA (ed) Water–Rock interactions, ore deposits, and environmental geochemistry. The geochemical soc special publication no. 7, pp 441–454
Hartzell P, Reed DW (2006) The genus archaeoglobus. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The prokaryotes. Springer, New York. https://doi.org/10.1007/0-387-30743-5_6
Hedin RS, Nairn RW, Kleinmann RLP (1994) Passive treatment of coal mine drainage. USBM IC 9389, Pittsburgh
Jarvis A, Gandy C, Bailey M, Davis J, Orme P, Malley J, Potter H, Moorhouse A (2015) Metal removal and secondary contamination in a passive metal mine drainage treatment system. In: Proceedings of 10th international conference on acid rock drainage (ICARD) and IMWA annual conference, April 21–24, Santiago, Chile
LaForce MJ, Colleen MH, Fendorf S (2002) Seasonal transformations of manganese in a palustrine emergent wetland. Soil Sci Soc Am J 66(4):1377–1389
Lewis-Russ A, Riese AC, Moore TJ, Brown AR (2022) Passive treatment of circumneutral mine drainage from the St Louis Mine Tunnel, Rico CO: part 1—case study: mine drainage characteristics. Mine Water Environ 41 (in this issue)
Li Y, Zheng X, Ma H, Hursthouse AS (2019) Removal of manganese(II) from acid mine wastewater: a review of the challenges and opportunities with special emphasis on Mn-oxidizing bacteria and microalgae. Water 11:2493
Luan F, Santelli CM, Hansel CM, Burgos WD (2012) Defining manganese(II) removal processes in passive coal mine drainage treatment systems through laboratory incubation experiments. Appl Geochem 27:1567–1578
Marsland R, Sobolewski A, Chandler T (2010) Tulsequah Chief passive bioreactor. In: Proceedings of 17th British Columbia MEND metal leaching/acid rock drainage workshop. Vancouver
Rose AW, Shah PJ, Means B (2003) Case studies of limestone-bed passive systems for manganese removal from acid mine drainage. In: Proceedings of annual society of mining and reclamation, pp 1059–1078
Skousen J, Zipper CE, Rose A, Ziemkiewicz PF, Nairn R, McDonald LM, Kleinmann RLP (2017) Review of passive systems for acid mine drainage treatment. Mine Water Environ 36(1):133–153
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851
US EPA (1993) Subsurface flow constructed wetland for wastewater treatment. a technology assessment, U.S. EPA 832-R-93-008, US EPA OWM, Washington, DC
Utgikar VP, Chen BY, Chaudhary N, Tabak HH, Haines JR (2001) Acute toxicity of heavy metals to acetate-utilizing mixed cultures of sulfate-reducing bacteria: EC50 and EC100. Environ Toxicol and Chem 20(12):2662–2669
Utgikar VP, Harmon SM, Chaudhary N, Tabak HH, Govind R, Haines JR (2002) Inhibition of sulfate-reducing bacteria by metals sulfide formation in bioremediation of acid mine drainage. Environ Toxicol 17(1):40–48
Widdel F (1988) Microbiology and ecology of sulfate- and sulfur-reducing bacteria. In: Zehnder AJB (ed) Biology of anaerobic microorganisms. Wiley, New York, pp 469–585
Widdel F, Bak F (1992) Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Truper HG, Dworkin M, Harder W, Schleifer KH (eds) The Prokaryotes, 2nd edn. Springer, New York, pp 3352–3378
Zhang M, Wang H (2016) Preparation of immobilized sulfate reducing bacteria (SRB) granules for effective bioremediation of acid mine drainage and bacterial community analysis. Miner Eng 92:63–71
Acknowledgements
The authors thank BP for access to the study site and for funding the research.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sobolewski, A.B., Riese, A.C., Moore, T.J. et al. Passive Treatment of Circumneutral Mine Drainage from the St. Louis Mine Tunnel, Rico CO: Part 3—Horizontal Wetlands Treatment Train Pilot Study. Mine Water Environ 41, 886–905 (2022). https://doi.org/10.1007/s10230-022-00856-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-022-00856-9