Abstract
A modelling methodology was developed that simulates the resulting pH and alkalinity in the mixing zone when acidic water is discharged into a river. The input to the model are the: pH, alkalinity, flow, and temperature of both the river water and the acidic discharge. Two different scenarios were simulated: (1) a change of pH in the acidic discharge, assuming constant flow; and (2) a change in the flow of the acidic discharge, assuming constant pH. The model incorporates the effect of carbonic acid and the modelled values agree well with the laboratory results. The model setup was subsequently used to predict the anticipated effect of contamination of the Zambezi River in Mozambique. The results indicate that the river will be impacted if the average pH of the water in the tributaries coming from the mining area is below 3. The model could be used by water managers to predict the potential impact of acidic discharges in poorly monitored rivers.
抽象
建立模拟酸性废水排入河流混水区的pH值和碱度模型。模型输入量包括:河水的pH、碱度、流量、温度及酸性废水的排放量。模拟了两种情形:(1) 假定酸性废水的排放量为定值而pH值改变;(2) 假定排放酸性废水的pH值为定值而排放量改变。模型考虑了碳酸的作用及影响。模拟结果与实验室结果非常吻合。应用该模型预测了莫桑比克Zambezi河污染及影响。结果表明,当源自矿区支流的平均pH值小于3时,河水水质将受影响。模型有助于预测排放酸性废水对缺少监测河流的影响。
Zusammenfassung
Es wurde eine Modellierungsmethode entwickelt, die den pH-Wert und die Alkaninität in der Mischungszone simuliert, wenn saures Wasser in einen Fluss eingeleitet wird. Die Eingangsdaten für das Modell sind: pH, Alkalinität, Durchfluss und Temperatur des Flusses und der sauren Einleitung. Zwei unterschiedliche Szenarien werden simuliert: (1) ein Wechsel des pH der sauren Einleitung unter Annahme konstanten Durchflusses und (2) ein Wechsel des Durchflusses der sauren Einleitung unter Annahme eines konstanten pH-Wertes. Das Modell berücksichtigt den Effekt des Karbonat-Systems. Die modellierten Werte stimmen gut mit Laborergebnissen überein. Das Modell wurde benutzt, um den Effekt der erwarteten Kontamination des Sambesi in Mosambique zu bestimmen. Die Ergebnisse zeigen, dass der Fluss geschädigt wird, wenn der mittlere pH-Wert der Zuflüsse aus Bergbaubereichen unter 3 liegt. Das Modell kann in der Wasserbewirtschaftung für die Vorhersage von Schädigungen von Flüssen durch saure Einleitungen bei geringer Monitoringintensität benutzt werden.
Resumen
Se desarrolló un modelo que simula el pH y la alcalinidad en la zona de mezclado cuando agua ácida se descarga en un río. Los datos de entrada para el modelo son: pH, alcalinidad, flujo y temperatura tanto del agua de río como de la descarga ácida. Se simularon dos escenarios diferentes: (1) un cambio de pH en la descarga ácida, suponiendo flujo constante y (2) un cambio en el flujo de la descarga suponiendo constante el pH. El modelo incorpora el efecto del ácido carbónico y los valores del modelo acuerdan con los resultados experimentales en el laboratorio. El modelo fue usado para predecir anticipadamente el efecto de contaminación del río Zambezi en Mozambique. Los resultados indican que el río será impactado si el pH promedio del agua en los tributarios que llegan a la zona minera, está por debajo de 3. El modelo podría ser usado para predecir el impacto potencial de descargas ácidas en ríos poco monitoreados.
Similar content being viewed by others
Introduction
Mining occurs in both developed and developing countries, bringing revenue to governments and improving people’s living standards though job opportunities (Nhantumbo et al. 2015). However, mining has also adversely affected the environment and water resources around the world (e.g. Anawar 2015; DPLF 2014; ICMM 2012; Ochieng et al. 2010). Reclamation of rivers and environments impacted by AMD is very expensive (Mishra et al. 2012); furthermore, developing countries often lack water quality monitoring programs to assess the impact of mining on their water resources (Nhantumbo et al. 2015). Financial and human resources limit the possibility of improving the water quality monitoring programs, if such programs even exist. Therefore, indirect methods, such as modelling, are an alternative way to predict water quality changes that allows for optimal allocation of resources required for water quality monitoring programs. Nevertheless, modelling cannot be used as a replacement to sampling and analysis and should be regarded as a complementary approach (Nordstrom 2012).
Over the years, there has been a plethora of models developed, such as Streeter-Phelps, QUAL, WASP, QUASAR, MIKE, BASIN, EFDC, OTIS, and PHREEQ C, aimed at simulating processes governing water quality in surface waters (Walton-Day et al. 2007; Wang et al. 2013). While these models can be put to good use, none of the models was actually developed to simulate water quality in rivers affected by acidification (Cardona et al. 2011; Munhoven 2013; Wang et al. 2013). PHREEQ C and OTIS can both be used to simulate mixing and transport of non-conservative pollutants in streams (Parkhurst and Appelo 2013; Walton-Day et al. 2007). PHREEQ C considers the effects of dilution when simulating mixing and has the capability to equilibrate the mixed solution to a given solid or gas phase (Parkhurst and Appelo 2013). Carbon dioxide (CO2), calcite (CaCO3), dolomite (CaMgCO3) and/or gypsum (CaSO4 · 2H2O) are the gas and solid phases commonly used (Parkhurst and Appelo 2013). The rationale for developing the present model is that dissolution of stream bed minerals and CO2 degassing are mass-transfer controlled processes that require a long time to evolve compared to the movement of water downstream in a river. Taking the example of CO2 degassing, the degassing coefficients can be in the range 10− 4–10− 6 m/s (Jiang and Wang 2008), whereas stream velocities typically are between 0.1 and 1.1 m/s (Benedini and Tsakiris 2013). As a result, equilibrium between the alkalinity species in surface water with CO2 in the atmosphere, and/or stream bed minerals, is only achieved some distance downstream of the mixing point. However, equilibrium between the alkalinity species that are dissolved in the water, such as H+, OH−, CO32−, HCO3−, and H2CO3, is reached in much shorter time. This might lead to lower pH values after the discharge of acidic water in the stream before CO2 degassing and dissolution of buffer minerals at the stream bed occurs to restore the pH.
When the OTIS model is used to simulate mixing in a stream, the only effect considered is dilution. Interaction between alkaline species that affects the pH is not considered (Walton-Day et al. 2007). Thus, there is a need to simulate pH in streams, considering both dilution and the equilibrium of alkaline species within the water. Such models should have three features: (1) transport, (2) adjustment of the equilibrium between the alkalinity species dissolved in the water (covered by the modelling methodology proposed in this paper), and (3) interaction of species in the water with the surrounding environment, inducing concentration changes towards equilibrium with the solid phase in the stream bed and the gas phase in the atmosphere (described using mass-transfer theories). Additionally, other limitations of water quality simulation models are that they typically require at least basic programming skills and/or large number of input parameter values, making them less appropriate for use in developing countries (Mosley et al. 2010, 2015).
The large number of rivers affected by acidification, the poor water quality monitoring programs in developing countries, and the lack of appropriate and simple models to simulate acidification required the development of a new modelling methodology that simulates water quality changes in rivers impacted by AMD, based on: (1) the mixing of the acidic discharge with the river water and (2) the reaction-transport processes taking place further downstream. The modelling methodology described in this paper assumes instantaneous complete mixing of the two streams and deals only with the chemical processes. It can be used to simulate and evaluate possible contamination of rivers by AMD or other acidic discharges, allowing for possible action to be taken to protect the water resources before severe consequences occur. However, the model is built is such a way that, in the future, it can be extended to simulate discharge of acidic water into lagoons or other water bodies.
Theoretical Development
The modelling methodology is based on alkalinity and the protolithic water theory. In the present approach, these two are combined to develop a simple and easy-to-apply model that can easily be extended to simulate a wide range of conditions pertaining to pH in the mixing zone of rivers affected by any acidic discharge. For the sake of simplicity and to allow easy demonstration of the methodology, this paper will consider only carbonaceous alkalinity. A more accurate model should include other alkaline species and the effects of iron, aluminum, and manganese.
Alkalinity
Alkalinity is defined as the ability of water to neutralize acids. It expresses the excess of proton acceptors over proton donors (Wolf-Gladrow et al. 2007). Alkalinity also reflects the excess of chemical bases of the solution relative to an arbitrary specified zero level, or equivalent point (Munhoven 2013). The expression to calculate total alkalinity (TA) is given by Eq. (1).
Ideally, the TA represents the amount of base contained in a sample of natural water that will accept a proton when a sample is titrated with a strong acid to the carbonic acid end point. The carbonic acid end point is the point where the hydrogen protons H+ become more abundant than hydrogen carbonate ions HCO3−. The carbonic acid end point is close to a pH of 4.3. AMD samples may present negative alkalinity, meaning that a strong base instead of a strong acid has to be added to reach the carbonic acid end point (Munhoven 2013).
Alkalinity, as defined above, is a conservative quantity with respect to mixing, as well as to changes in temperature and pressure (Wolf-Gladrow et al. 2007). Normally, in natural waters, carbonate alkalinity is the most important part of the TA. Equation (1) can therefore be reduced to Eq. (2).
The carbonic acid is directly related to the pH of surface water. The equilibrium of carbonic acid in water is defined by the Eqs. 3–6 (Appelo and Postma 1999).
The equilibrium constants are temperature dependent and there are empirical equations already developed to describe this dependence. The empirical relationships are given by Eqs. 7 and 8 (Appelo and Postma 1999):
The Total Inorganic Carbon
The total inorganic carbon (TIC) is the sum of the inorganic carbon species in natural water. The TIC can be calculated using Eq. (9).
The TIC is also a conservative quantity with respect to mixing in water, but not necessarily with regard to temperature and pressure. The concentration of each carbonate ion in the TIC can be calculated when the TA and pH of a water are known by combining Eqs. 2, 4 and 6, yielding Eqs. (10–12).
Protolithic Theory of Water
The dissociation constant of water determines the relationship between molar concentration of H+ and OH− in water at a specific temperature. Equations 13 and 14 show the chemical and mathematical interpretation.
For convenience, the concentration of H+ is usually expressed in terms of pH, Eq. 15.
The dissociation constant of water can be estimated using the temperature-dependent empirical Eq. 16 (Appelo and Postma 1999).
Procedures
A modelling methodology for simulating the pH and alkalinity of streams affected by an acidic discharge was developed based on the alkalinity and protolithic water theory described above. The input data to the model are the pH and alkalinity of the two streams that are mixing, the natural river water flow and the acidic discharge. The modelling methodology was validated by: (1) evaluating the model’s results, considering the buffer effect of carbonic acid in water and (2) comparing the modeled values with laboratory data. Furthermore, an example of model application is presented using data on flow, alkalinity, and pH from the Zambezi River and its tributaries that are at risk of being impacted by AMD from coal mining in Mozambique.
Model Development
The volumetric flows in the model are denoted by Q1, Q2, and QR for the upstream river, acidic discharge, and downstream river, respectively (Fig. 1). The flows can be replaced by volumes when simulating acidification of lagoons, or other similar water bodies. The model was formulated assuming complete and instantaneous mixing, that is, a completely stirred (CS) mixing zone. This may not be a true representation of what happens in reality for large rivers; however, it give a reasonable first approximation, or at least a worst case scenario, of resulting downstream concentrations. A more complete model would require an analysis to resolve spatial variation. The model is based on the assumption that when two streams, Q1 and Q2, are mixed, the TIC and total alkalinity (TA) can be added in moles, because these two quantities are conservative during mixing. The resulting total alkalinity (TAR) and total inorganic carbon (TICR) can be calculated using Eqs. 17 and 18, respectively.
The concentration of each carbonate specie can be calculated using Eqs. 19–21.
The values of \(\alpha _{{H_{2} CO_{3} }}\), \(\alpha _{{HCO_{3}^{ - } }}\), and \(\alpha _{{CO_{3}^{{2 - }} }}\) can be made functions of [H+] by using Eqs. 4, 6, 9, 19, 20, and 21; see Eqs. 22–24.
The TAR can also be calculated using the definition, Eq. 2. The TAR calculated using this definition has to be equal to the TAR calculated using the sum of the alkalinity of the two streams (\(TA_{{R{\prime }}} = TA_{R}\)). Thus, Eq. 25 is obtained by combining Eqs. 2, 19–24.
Equation 25 can be solved using a numerical method, for example Newton–Raphson (N–R), to obtain the concentration of H+. In N–R, if there is a function \(f([{H^+}])=0\), an iterative approach is used to determine consecutively improved values of concentration of H+, employing Eq. 26.
The function \(f([{H^+}])\) and its derivative \({f^\prime }([{H^+}])\) are given by Eqs. 27 and 28, respectively.
The calculation steps of the modelling methodology are given in the flow chart (Fig. 2). The input values to the model are the upstream river flow and acidic water discharge, the pH and TA of both flows, and the average temperature of the water. The output of the model are the TA and pH of the resulting stream (TAR and pHR), as well as the carbonate ion speciation.
Flow diagram of the model that estimates the pH and the resulting alkalinity (pHR and TAR) from the mixing of two streams with different pH and alkalinity. The model is based on input of flows of the streams (Q1 and Q2), pH of the streams (pH1 and pH2), alkalinity of the streams (TA1 and TA2), and the average water temperature (T). The model uses as a default value for temperature of 25 °C, but it can be adjusted if necessary. A function f([H+]) = 0 obtained from the theories of carbonate speciation and pH in water is solved using the Newton–Rapson method. The output of the model are pH and TA of the resulting stream (pHR and TAR), as well as the carbonate ions speciation
Laboratory Experiments
To support the current research work, six experiments were performed yielding validation data for the model (Table 1). The model was run to simulate the pH and alkalinity for a river in the mixing zone considering: (1) a change of volumetric flow in the acidic discharge while pH remained constant and (2) a change in pH of the acidic discharge while the volumetric flow remained constant. In both cases, the main stream river flow was assumed to be constant.
To validate the model for the two simulation scenarios, two different contamination conditions were created: in the first contamination condition, which is expected to be more ideal, deionized water was used to prepare all of the water samples, whereas in the second contamination condition, tap water from Sydvatten, the company that supplies water to the municipality of Lund was used to prepare the samples. Either hydrochloric acid (HCl) or sulfuric acid (H2SO4) was used to lower the pH, NaOH was used to increase the pH, and Na2CO3 was used to increase the alkalinity to the values indicated in Supplemental tables 1–6.
Alkalinity was determined by automatic titration (Burette Digital - BRAND, 25 mL, with an error of ± 0.005 mL) with solutions of HCl, for water with a pH > 4.3, and NaOH for water with a pH < 4.3. The pH end point used for the titration was 4.3. The concentration of the titration solution used was 0.1 N for experiments 1, 2, 3, and 4, and 0.05N for experiments 5 and 6, for both HCl and NaOH (Table 1). The pH was measured using a pH meter (pH 320 by WTW, with an error of ± 0.005). The volumes of the samples for the experiments were measured using 25 and 50 mL pipettes, equipped with rubber pipette fillers, having an error of ± 0.25 mL.
Initial water samples of acidic water were prepared using concentrated HCl (EMSURE 37% w/w) or H2SO4 (EMSURE 95–97% w/w). Initial solutions of water with a high pH were prepared using pellets of NaOH (EMSURE 99% w/w) and the alkalinity was increased with Na2CO3 powder (EMSURE 99.9% w/w). The pH and alkalinity of the initial solutions were further measured using the pH meter and titration to get actual values.
The experiments to validate the first simulation scenario were carried out using two levels of alkalinities, low alkalinity, experiments 1 and 3, and high alkalinity, experiments 2 and 4 (Table 1). In the text below, when two water samples are mixed; basic water is the one with a high pH and acidic water the one with a low pH.
The initial conditions of the water samples used in the laboratory together with the measured pH and alkalinity after mixing acidic and basic water for experiments 1–6 are given in Supplemental tables 1–6. The modeled results were compared with the laboratory measurements using the correlation coefficient (R) and the associated significance level, the p-value, and the Nash–Sutcliffe Efficiency (NSE), Eq. 29. The correlation coefficient R is considered significant only if the associated p-value is below 0.05.
where: n is the total number of observations, \(y_{i}^{{meas}}\) is the value measured in each experiment, \(y_{i}^{{mo}}\) is the corresponding modeled value, and \(\overline {{{y^{meas}}}}\)is the average of the experimental values (Taylor et al. 2016).
Thus, NSE describes the “goodness-of-fit” between the experimental and modeled values. It can vary from − ∞ to 1, where the value of 1 represents a perfect fit. A value between 0 and 1 is generally recognized as acceptable model performance, whereas a value less than zero means that the average of the measured values is a better predictor of a variable compared to the model, indicating unsatisfactory model performance (Taylor et al. 2016).
Results and Discussion
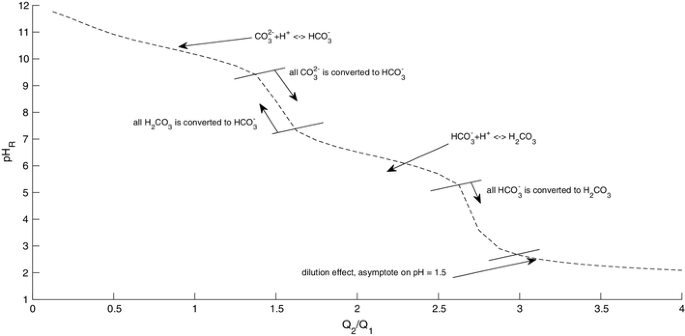
The model was tested for the mixing of two hypothetic streams with the flows Q1 and Q2 to evaluate if modeled values correctly represent the buffer effect of carbonic acid in water. A broad range of pH (12–1.5) was used to evaluate the model performance for high, medium, and low pH values. An alkalinity of 0.086 eq/L was used to allow for buffer effect visualization.
Figure 3 shows that as the flow of acidic water (Q2) increases, the pH of the resulting stream, pHR tends to remain constant in three different zones, between pH 9–12, 5–7, and 1.5–3. Between these three sections, there are two intervals where the pH drops rapidly with the addition of small amounts of acidic water Q2. The two zones where the pH drops with a small addition of acidic water shows the total conversion of CO32− to HCO3− and HCO3− to H2CO3, respectively. The first two zones of Fig. 3, where the pH tends to remain constant when the acidic water is added, is due to the buffering effect of carbonic acid, whereas the third section is an effect of the dilution, reflecting that the pH of the resulting stream tends to be equal to the pH of the acidic stream, Q2. These results show that the model can capture the effect of carbonic acid buffering.
The concentrations of carbonate species (H2CO3, HCO3−, CO32−), TA, and TIC was as expected (Fig. 4). The TIC at any pH is the sum of the inorganic species but it diminishes with pH reduction. This is because the TIC in the stream Q2 is lower than the TIC in the stream Q1. The TA decreases as the pH falls, reaching zero at pH 4.3. After pH 4.3, the alkalinity has been totally consumed. The negative value of TA shows that the water is getting even more acidic.
Concentration of carbonic acid (H2CO3), bicarbonate (HCO3−), carbonate (CO32−), TICR, and TAR vs pHR in eq/L. pHR is the pH of the stream resulting from mixing of Q1 and Q2, where Q1 (pH = 12, alkalinity = 0.086 eq/L) and Q2 (pH = 1.5, alkalinity = − .032 eq/L) are from two streams mixed with different proportions
Comparison Between Model and Laboratory Results
Figures 5 and 6 show the predicted and measured values for the first simulation scenario using (1) deionized water and HCl, and (2) tap water and H2SO4, respectively, for scenarios with low and high alkalinity. The figures show that the modelled pH and alkalinity agree with the measured values. However, the measured alkalinity in the experiments with tap water and H2SO4 was below the alkalinity estimated by the model. In the high alkalinity experiment, the difference is about 0.02 eq/L at higher pH values (Fig. 6b). This difference is due to the HSO4− introduced by the sulfuric acid that was used to produce acidic water in the experiments, which was not considered in the model, see Eq. 1.
Model prediction and measured values of a pH and b alkalinity. V2 is the volume of acidic water (representing acidic discharge) and V1 is the volume of basic water (representing the river water before contamination). pHR is the pH resulting after mixing V1 and V2. The laboratory experiments were performed using two levels of initial alkalinity (V1) corresponding to 0.0093 and 0.054 eq/L. The experiments were done using dionized water, HCl to lower the pH, NaOH to increase the pH, and Na2CO3 to increase the alkalinity
Model prediction and measured values of a pH and b alkalinity. V2 is the volume of acidic water (representing acidic discharge) and V1 is the volume of basic water (representing the river water before contamination). pHR is the pH resulting after mixing V1 and V2. The laboratory experiments were done using two levels of initial alkalinity for V1 corresponding to 0.012 and 0.033 eq/L, respectively. The experiments were done using tap water, H2SO4 to lower the pH, NaOH to increase the pH, and Na2CO3 to increase the alkalinity
Figures 7 and 8 show the modeled and measured values for the second simulation scenario using (1) deionized water and HCl, and (2) tap water and H2SO4, respectively. The figures show that the modelled pH and alkalinity agree with the measured values. In the changing pH experiment, the modeled and measured alkalinity deviate at low pH (Fig. 8b). This happens because experiments were performed differently. For the changing pH experiments, the initial samples were both prepared with a high pH, while for the changing volume experiments, one of the initial samples were prepared with a high pH and the other with a low pH (Supplemental tables 4, 5, and 7). For the changing pH experiments, the pH was lowered by adding acidic water (prepared with H2SO4) to lower the pH to V2.
Model prediction and measured values of a pH and b alkalinity resulting when constant volumes of acidic water (representing acidic discharge) and basic water (representing the river water) are added varying the pH of the acidic water. pHR is the pH of the water sample resulting from adding basic and acidic water. pH2 is the pH of the acidic water. The experiment were done using deionized water, HCl to lower the pH, NaOH to increase the pH and Na2CO3 to increase the alkalinity
Model prediction and measured values of a pH and b alkalinity resulting when constant volumes of acidic water (representing acidic discharge) and basic water (representing the river water) are added varying the pH of the acidic water. pHR is the pH of the water sample resulting from adding basic and acid water. pH2 is the pH of the acidic water. The experiments were done using tap water, H2SO4 to lower the pH, NaOH to increase the pH, and Na2CO3 to increase the alkalinity
The correlation coefficients in all of the experiments were significant at a confidence level of 0.95 (Table 2). Calculated p-values for all correlation coefficients were much less than 0.05. NSE values were between 0 and 1 for all experiments. The correlation coefficient together with the NSE values reveal that the model is accurate enough to be used for practical applications (Table 2). An example of such a practical application is given below, given to illustrate how the model can be used in practice.
Applying the Model to the Zambezi River in Mozambique
The Zambezi River Basin is the largest river basin in southern Africa, with a surface area of 1,370,000 km2 and an average discharge at the outlet of 4100 m3/s. It sustains life for about 30 million people and is essential for the economy of its riparian countries, which include Angola, Botswana, Malawi, Mozambique, Namibia, Tanzania, Zambia, and Zimbabwe (Supplemental Fig. 1; Nhantumbo et al. 2015).
Over the past 10 years, coal mining has been increasing along the Zambezi River Basin, with the associated threat of severe impacts to water quality. However, these impacts can be minimized if there is a well-developed water quality monitoring program to allow early detection of negative trends and an understanding of actions that can be taken to protect the water resources. However, water quality monitoring is not well established in the Zambezi River Basin and a lack of resources limits its improvement (Nhantumbo et al. 2015). Predicting the acidity level of the water coming from the main river tributaries would potentially allow water managers to implement targeted water quality monitoring programs and better protect the watershed.
The three main coal reserves (Chicôa-Mecúcoè in the west, Sanângoè-Mefídezi in the southwest, and Moatize-Minjova in the northeast) of Mozambique are in the riparian area of the Zambezi River Basin in Tete Province (Fig. 9) (Nhantumbo et al. 2015). Several mining companies are exploring and some are already mining coal in Tete. The most exploited reserve is the Moatize-Minjova in the Moatize district (Nhantumbo, Larsson, Juizo, & Larson, 2015). Static and leaching tests indicate that there is a significant probability of having AMD in the Moatize district (Pondja et al. 2016). The tributaries of the Zambeziet al. Basin passing through the Moatize district (the Revúbue, Mavuzi, and Condadezi Rivers) are the most likely to be impacted by mining. Open pit mining is currently being used to exploit coal in Moatize; the Moatize-Minjova is a karoo-aged rift characterized by interbedded carbonaceous mudstones and sandstones, together with coal seams (Pondja et al. 2016). Water samples collected from tet al.e pits had a near-neutral pH and a high content of sulfate, calcium, and magnesium, which is indicative of neutralization of the acid by carbonates and silicates (Pondja et al. 2016).
Coal mining in Tete Province in Mozambique (Nhantumbo et al. 2015)
The average flow, pH, and alkalinity of both the main stream and the tributaries from the mining area are listed in Table 3. At present, there is no contamination reported in the main stream that indicates acidification (Nhantumbo 2013). Our task was to determine the threshold pH of the effluent from the mining area that would trigger significant changes in the Zambezi River. To address this question, we used the model developed in the present study to simulate the change in pH in the Zambezi River that would result from a change in pH of water from the mining area. For the simulation, the pH in the effluent from the mining area was assumed to range from 7.85 to 2.
The results of the simulation indicated that the pH in the main stream of the Zambezi River would drop from about 6 to 3 when the average pH of the water in the incoming tributaries from the mining area was about 3 (Fig. 10a). This shows that with the present alkalinity and flows, the main stream of the Zambezi River cannot support a drop in pH in water from the mining area to below 3. Additional analysis was done using the speciation of the carbonate ions, TIC, and TA (Fig. 10b). By comparing Fig. 10a, b, one can see that the pH drops when the alkalinity in the main stream is totally consumed.
The pH of the water coming from the mining area must not drop below 3 to avoid significant harm to the river. However, depending on the geology of the area, even water with a pH above 3 might contain enough contaminant metals to adversely affect the water of the main stream, even though the pH remains above 6.5. Also, the model considers only the carbonaceous alkaline species. The effects of other alkaline species and the hydrolysis and precipitation of iron and aluminum, which would likely significantly affect the pH, were not considered. Thus, the simulated results can only be considered to be a first approximation. Nevertheless, the modelling methodology is flexible, making it possible to include the effects of other alkaline species, and metal hydrolysis and precipitation. For more detailed modelling, it may be desirable to extend the model to include these effects.
At least some CO2 degassing will take place and there is a chance that buffering mineral in the bottom of the stream might dissolve; all these effects will reduce the impact. There is also a chance that zonation will create localized places with extremely low pH (Schemel et al. 2006), but this effect can only be understood using three-dimensional modelling, which was not attempted in this study.
Model Potential Applications, Limitations, and Opportunities for Improvement
At present, the model can be applied to simulate different contamination scenarios as river water and acidic discharges mix. In practice, the model can be used as a decision support tool by authorities for granting new mining licenses and by different industries that produce acidic wastewaters to manage their discharges to the environment. However, modelling results should only be used to approximate the environmental impact in order to limit exploitation of a certain resource or the discharge of acidic water. As previously pointed out, relying only on modelling results is not recommended (Nordstrom 2012). Water quality monitoring must be implemented during the operational phase.
The model requires a limited amount of input data: the flow of the river and the acidic discharge, the pH and alkalinity of both streams, and the average temperature of the water. This makes the model useful for estimating the pH and alkalinity in remote areas where there is lack of resources and data.
At the present stage of model development, there are some limitations that need to be considered before practical applications: (1) the model considers molar concentrations, not activities, and it can only be used at sites where the ionic strength is low as indicated by TDS being below 1000 mg/L or electrical conductivity below 1500 µS/cm; (2) only carbonaceous species alkalinity is considered and the effect of other species, especially iron, manganese, and aluminum concentrations, may affect the results of the model when dealing with water having a pH < 4.3; (3) the model assumes instantaneous mixing, which is only an approximate assumption and may limit the applicability of model when accurate values of pH are needed (mixing in natural rivers can be affected by river width, depth, water velocity, roughness of the stream bed, and the relationship between the flow of the main stream and the acidic discharge); hence the time needed to have complete mixing and equilibrium of the alkalinity species in the water might be longer then considered in the model; (4) the model cannot capture zonation in wide and deep streams; and (5) the model should only be used when the purpose is to investigate the pH of rivers impacted by acidic discharge and no detailed information about the concentrations of metals are required.
The approach can be improved by: (1) using activities other than molar concentration to extend the model’s applicability to high ionic strength waters; (2) including more alkaline species as well as the effects of iron, manganese, and aluminum in Eq. 27; (3) investigating the effects of stream width, depth, water velocity, stream bed roughness, and the relationship between the flow of the main stream and the acidic discharge, as well as the effect of CO2 degassing and dissolution of stream bed minerals, to obtain the mixing zone length and the minimum pH value at the mixing zone; (4) including three-dimensional transport in the mixing zone; and (5) including simulation of metal concentrations.
Nevertheless, the model, despite its limitations, can be used to make a preliminary evaluation of the impact of acidic discharges into rivers. Some of the main advantages of the model are its simplicity and minimal data requirement. The approach can be further developed to simulate extremely low pH cases, such as Iron Mountain in California (Nordstrom et al. 2000), by expanding Eq. 27, as mentioned above.
The model does not simulate what happens downstream of the discharge point because the model does not simulate the necessary reaction and transport processes that take place downstream. However, the methodology opens up the possibility of developing models that include both near- and far-field transport and mass transfer features when acidic water is discharged into a stream because it uses a different approach than other currently available models. Equilibrating the alkaline species within the water with certain gas and solid phases, as it is done in PHREEQC, only simulates the pH and alkalinity accurately far downstream in surface waters, where the solid and gas phases have already reached equilibrium. At the same time, using only the effect of dilution, like the OTIS model, can introduce errors in the estimated concentrations when the water chemistry is complex. When acidic water is discharged into a stream, the equilibrium between the species within the water is immediately reached, but the equilibrium with the stream bed solid phase and the atmospheric gases is reached further downstream. The methodology suggested in the paper only considers the equilibrium of aqueous species within the water and mass transfer theories that can be used to simulate the exchange of alkaline species with the surrounding environment as the water travels downstream. Further development of the model should include features to simulate the reaction and transport-process in the river water, which are the main constraints of available models in simulating pH and alkalinity (Mosley et al. 2010, 2015).
Conclusions
A model that simulates the pH and alkalinity at the mixing point in a river affected by an acidic discharge was developed based on theoretical understanding of alkalinity and pH in natural waters. A nonlinear equation in terms of the concentration of hydrogen ions was obtained and solved using the Newton–Raphson technique to get the concentration of hydrogen ions. The model can simulate two different contamination scenarios in the mixing zone: (1) constant pH of the acidic discharge but a varying flow and (2) constant flow of the acidic discharge but a variable pH. The model was evaluated by its agreement with the buffering provided by carbonic acid in water and validated by comparison with data from laboratory experiments.
The modeled results agreed well with the buffering effects of carbonic acid in water. The correlation coefficients between the modeled and measured data were always above 0.8 and the related p-values were always below 0.05. This means that the model is accurate enough to be used for practical applications.
Furthermore, the usefulness of the model was shown using data from the Zambezi River in Mozambique. From the simulated results, it was concluded that a significant drop in the pH of the main stream of the river will be registered only if the pH of the tributaries drops below 3.
The model can be further developed to simulate the reaction transport process taking place further downstream of the mixing zone. Such an improvement would allow the model to simulate natural recovery, and allow it users to plan the treatment as well as to monitor the reclamation of the already impacted stream.
References
Anawar H (2015) Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J Environ Manage 158:111–121
Appelo CA, Postma D (1999) Geochemistry, groundwater and pollution. AA Balkema, Rotterdam
Cardona C, Martin C, Salterain A, Castro A, Martin A, Ayesa A (2011) New software application for river quality prediction based on RWQM1. Environ Modell Software 26:973–979
Cowie R, Williams MW, Wireman M, Runkel RL (2014) Use of natural and applied tracers to guide targeted remediation efforts in an acid mine drainage system, Colorado Rockies. USA Water 6:745–777
DPLF (2014) The impact of Canadian mining in Latin America and Canada’s responsibility. Duo Process of Law Foundation (DPLF)—Working Group on Mining and Human Rights in Latin America, vol 1. Washington, pp 1–40
ICMM (2012) Water management in mining: a selection of case studies. International Council on Mining and Metals. https://www.icmm.com/website/publications/pdfs/water/water-management-in-mining_case-studies. Accessed 1 June 2016
Mishra SK, Hitzhusen FJ, Sohngen BL, Guldmann JM (2012) Costs of abandoned coal mine reclamation and associated recreation benefits in Ohio. J Environ Manage 100:52–58
Mosley L, Peake B, Hunter K (2010) Modelling of pH and inorganic carbon speciation in estuaries using the composition of the river water and seawater end members. Environ Modell Softw 25:1658–1663
Mosley L, Daly R, Palmer D, Yeates P, Dallimore C, Biswas T, Simpson S (2015) Predictive modelling of pH and dissolved metal concentrations and speciation following mixing of acid drainage with river water. Appl Geochem 59:1–10
Munhoven G (2013) Mathemathics of the total alkalinity -pH equation - pathway to robust and universal solution algorithms: the SolveSAPHE package v1.0.1. Geochem Model Dev 6:1367–1388
Nhantumbo C (2013) Evaluation of Long-term Impact of Coal Mining in Zambezi River Basin in Mozambique. Lund University Publications, Lund
Nhantumbo C, Larsson R, Juizo D, Larson M (2015) Key issues for water quality monitoring in the Zambezi River Basin in Mozambique in the context of mining development. J Water Resour Prot 7:430–447
Nordstrom DK, Alpers CN, Ptacek CJ, Blowes DW (2000) Negative pH and extremly acidic mine waters from iron mountain California. Environ Sci Technol 34:254–258
Nordstrom DK (2012) Models, validation, and applied geochemistry: Issues in science, communication, and philosophy. Appl Geochem 27:1899–1919
Ochieng GM, Seanego ES, Nkwonta OI (2010) Impacts of mining on water resources in South Africa: A review. Sci Res Essays 5:3351–3357
Olías M, Nieto JM (2015) Background conditions and mining pollution throughout history in the Río Tinto (SW Spain). Environments 2:295–316
Parkhurst DL, Appelo C (2013) Description of input and examples for PHREEQC Version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Retrieved December 14, 2016 from https://pubs.usgs.gov/tm/06/a43/pdf/tm6-A43.pdf
Pondja E, Persson K, Matsinhe N (2016) Proposal of standard guidelines for effluent water in coal mine in Mozambique. Int J Manage Appl Sci 2:28–33
Schemel LE, Cox MH, Runkel RL, Kimball BA (2006) Multiple injected and natural conservative tracers quantify mixing in a stream confluence affected by acid mine drainage near Silverton, Colorado. Hydrol Process 20:2727–2743
Svitok M, Novikmec M, Bitušík P, Máša B, Oboňa J, Očadlík M, Michalková E (2014) Benthic communities of low-order streams affected by acid mine drainages: A case study from Central Europe. Water 6:1312–1338
Taylor SD, He Y, Hiscock KM (2016) Modelling the impacts of agricultural management practices on river water quality in Eastern England. J Environ Manage 180:147–163
Walton-Day K, Paschke SS, Runkel RL, Kimball BA (2007) Using the OTIS Solute-Transport Model to evaluate remediation scenarios in Cement Creek and the Upper Animas River. In: Church SE, Guerard P, Finger SE (eds) Integrated Investigations of Environmental Effects of Historical Mining in the Animas River Watershed San Juan County. U.S. Geological Survey Professional, Colorado, p 1651
Wang Q, Li S, Jia P, Qi C, Ding F (2013) A review of surface water quality models. Sci World J 2013:1–7
Wolf-Gladrow DA, Zeebe RE, Klaas C, Körtzinger A, Dickson AG (2007) Total alkalinity: the explicit conservative expression and its application to biochemical processes. Mar Chem 106:287–300
Acknowledgements
The research was supported by the Swedish International Development Cooperation (SIDA) under agreement EMU-SIDA 2011–2015. We thank Carlos Lucas, the Head of the Cooperation Department, and Nelson Matsinhe, the coordinator of the Water Quality Programme, at Eduardo Mondlane University (EMU), for their patient assistance. We are also grateful for the monthly stipend given by the Lars Erik Lundberg Scholarship Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nhantumbo, C., Larsson, R., Larson, M. et al. A Simplified Model to Simulate pH and Alkalinity in the Mixing Zone Downstream of an Acidic Discharge. Mine Water Environ 37, 552–564 (2018). https://doi.org/10.1007/s10230-018-0515-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-018-0515-3