Abstract
Anadromous migration of salmonid fish has been extensively studied, primarily focusing on the dichotomous framework of anadromy and residency. However, there remains a limited understanding of intermediate migratory behaviors within the anadromous framework. Our study aimed to classify the lifetime migration patterns of the anadromous white-spotted charr Salvelinus leucomaenis within and among populations using otolith annuli and Sr:Ca ratios. Initially, the migratory histories of anadromous charr were divided into two stages: “virgin sea-run stage” and “veteran sea-run stage”. The former was further categorized into three types: ocean entry at age ≥1+ years old, early descending, and brackish use. The latter was grouped into four types: annual migrants, frequent migrants, retired migrants, and ocean residents. We found that the proportion of migration patterns varied among rivers, with multiple patterns coexisting within the same river. Migration patterns typically involving 1–5 years spent in freshwater rivers followed by annual oceanic migrations were the most abundant, although diverse patterns were also observed. In the virgin sea-run stage, some individuals experienced the ocean at age 0+ years or brackish environments before their first sea entry. In the veteran sea-run stage, we found individuals who had resided in either the ocean or rivers for over a year. Retired migrants, characterized by stopping oceanic migration at a certain age and subsequently spending time in rivers, were exclusive to southern rivers. Conversely, ocean residents who spend one or more years in the ocean were more frequent in northern regions. Consequently, the lifetime migration patterns of anadromous white-spotted charr may exhibit stronger ocean dependency at higher latitudes. The implications of this study highlight the complexity and flexibility of migratory behaviors within and among white-spotted charr populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salmonids are a representative and well-studied group of anadromous fish with approximately 100 known species (Quinn 2021). Anadromous salmonids migrate over long distances and periods, whereas resident salmonids complete their entire life cycle in freshwater streams (Jonsson and Jonsson 1993). Consequently, previous works have largely examined their migratory strategies within dichotomous frameworks. Recent studies, however, have attempted to explore the diversity within migratory communities on a continuum. Terms like “migration continuum”, “life-history contingent”, or “differential migration” have been introduced to describe some intermediate patterns where individuals vary in distance or timing of migration (Cucherousset et al. 2005; Quinn 2021; Zimmerman et al. 2022). For example, brown trout Salmo trutta exhibit a migration continuum that varies across ranges, ages, and cohorts, mainly in response to environmental changes (Cucherousset et al. 2005). Precocious emigrants, in which young parr shift their habitat to brackish water without smoltification, appear in short streams (Limburg et al. 2001). Additionally, autumn outmigrants make use of safe but unproductive brackish waters (Wynne et al. 2023). Such life-history diversity in portfolios may make the population more resilient to global changes (Price et al. 2021). The extent of anadromy varies among different salmonid species (Rounsefell 1958; Quinn and Myers 2004). The genera Oncorhynchus, Salmo, and Salvelinus are arranged in descending order of marine adaptation, suggesting that recently evolved salmonids are more adapted to marine life histories (Spares et al. 2015). While Klemetsen (2013) has emphasized that the Arctic charr Salvelinus alpinus is the most variable vertebrate on Earth with potentially diverse migratory ecology; however, ocean migration in Salvelinus species remains understudied (Spares et al. 2015).
The white-spotted charr Salvelinus leucomaenis is distributed from Kamchatka to northern Japan in East Asia. They are anadromous iteroparous species with complex life cycles and migratory patterns. The typical life history of white-spotted charr has been studied in Hokkaido, Japan, as summarized by Sahashi and Morita (2024). These charr spend several years in their natal rivers until reaching smolt age, which ranges from 2+ to 7+ years (Yamamoto and Morita 2002). After smolting and descending to the sea, they feed in the ocean for 1.5–4 months during the summer. They then return to freshwater rivers for either spawning in the fall or overwintering. This cycle of feeding migrations to the sea, spawning, and overwintering in rivers is repeated annually. However, migration patterns are not uniform, and exceptional timing, such as non-annual short-term migration, has been reported (Takami et al. 1996; Arai and Morita 2005; Morita et al. 2013; Kuroda and Miyashita 2022). In addition, the white-spotted charr migrates a relatively short distance from the natal river mouth (Takami 1995; Aoyama 1997).
Northern Japan, serves as an ideal model system for studying geographic variation in anadromous white-spotted charr. These islands encompass a broad range of latitudes, and the Tsushima Warm Current dominates the Sea of Japan, providing environmental gradients along latitudes, such as water temperature. Previous research has shown that the life history traits of white-spotted charr exhibit latitudinal trends within this geographic range (e.g., higher latitude and larger body size, Maekawa and Nakano 2002; smaller smolt size and older age, Yamamoto and Morita 2002; higher proportion of anadromous form, Yamamoto et al. 1999a; less feeding in the river, Goto et al. 2023). Gross et al. (1988) noted that anadromous fishes are more common at higher latitudes, where marine productivity is higher than that in the surrounding freshwater areas, suggesting oceanic dependence on latitudinal clines in relative productivity of diadromous fishes. Similar to the patterns of occurrence of anadromous species and individuals, it is also assumed that latitudinal trends may be observed in diadromous migratory patterns even within the anadromous individuals, but this has not been verified yet. Therefore, studying geographic variations in migratory patterns using widely distributed fish species with limited migratory range can offer insights into these latitudinal clines in oceanic dependence within species.
The aim of this study is to clarify the migratory diversity of anadromous white-spotted charr within and among populations. We hypothesize that ocean-dependent migration patterns will appear more frequently within populations at higher latitudes. Specifically, we examine (1) the classification of lifetime migration patterns using otolith microchemistry analysis and (2) the geographical trends in the migration patterns of anadromous white-spotted charr.
Materials and methods
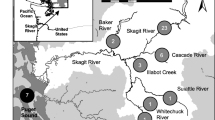
Fish collection. We collected white-spotted charr from 16 rivers flowing into the Sea of Japan, located on the Japanese archipelago (46°26′N–38°06′N). This includes rivers in Hokkaido, the Honshu Islands, and surrounding smaller islands [Electronic Supplementary Material (ESM) Table S1; Fig. 1]. Sixteen rivers were grouped into three river groups (northern Hokkaido, 5 rivers; southwestern Hokkaido, 6 rivers; and Honshu, 5 rivers) based on the influence of the Tsushima Warm Current. These regions represent the southernmost extent of anadromous charr distribution. We selected 16 rivers for sampling with depths suitable for electrofishing, river mouth widths of approximately <10 m, and flow lengths of <10 km. Survey areas in these rivers were selected to exclude sections with migration barriers such as dams or waterfalls, allowing white-spotted charr the potential for diverse migratory patterns as they can move freely between the sea and rivers. River mouth widths, water temperatures, and migration barriers were measured during the survey. The gradient of each river was calculated using the distance to the 20-m altitude point on 1:25,000 scale topographic maps (http://maps.gsi.go.jp). Catchment areas were also calculated on 1:25,000 scale topographic maps.
Fish were captured using an electrofisher (300–400 V DC, model 12 B, Smith-Root Inc., WA, USA) and hand nets during August and September of 2019 and 2020, when most of the sea-run migrants were returning to river. We focused on collecting potential sea-run migrants, specifically individuals larger than 20 cm, lacking parr marks, and displaying a silver body color (ESM Fig. S1). Some of the specimens were sourced from the study by Goto et al. (2023).
All captured white-spotted charr were measured onsite for fork length (FL, mm) in 1 mm increments and body weight (BW, g) in 0.5 g increments up to 1,000 g and in 1 g increments for individuals weighing over 1,000 g (ESM Table S2). Condition factor (CF) was calculated by BW/FL3 × 106. Following dissection, the sex of each individual was identified through visual observation of the gonads. Sagittal otoliths were extracted from each fish and preserved in 99% ethanol for subsequent age and migration history analysis.
Otolith analyses. The age of each fish was determined by analyzing the otolith annuli (opaque) formed from late May to July each year, as described by Yamamoto et al. (1992). Because white-spotted charr hatch in winter, the opaque closest to the core was considered the young-of-the-year annulus (age 0+). Otolith images were captured using a stereomicroscope (SMZ1500, Nikon, Japan) coupled with a camera (DS-Fi1, Nikon, Japan) under reflected light against a black background. Using ImageJ (ver.1.52), we measured the otolith radius and the distance from the core to each annulus in 1 μm increments along the maximum radius from the outmost core of coagulated multiple foci to the posterior edge.
Following age determination, otoliths were embedded in epoxy resin (EpoFix, Struers, Denmark) and mounted on glass slides for trace element analysis to identify migratory patterns. The otoliths were ground using SiC papers (#800–4,000) on a polishing machine (RotoPol-35, Struers, Denmark) until the core was exposed, then polished with OP-S suspension (Struers, Denmark). After washing and drying, the otoliths were coated with Pt-Rd for 60 s using an ion sputter (E-1030, Hitachi, Japan). To analyze Sr and Ca, electron microprobe analyses were performed using an electron probe microanalyzer (EPMA; JXA-8230, JEOL, Japan) at an acceleration voltage of 15 kV and a current value of 12 nA. The measurement diameter was 9 μm, and the interval was 10 μm from the core to edge along the same transect used in age analysis. To estimate migration history, we used the value calculated as Sr:Ca × 1,000 (wt%/wt%) from the measured Sr and Ca (hereafter referred to as the Sr:Ca ratio). Two-dimensional X-ray intensity maps of Sr and Ca were obtained for some specimens using the EPMA under the following measurement conditions: acceleration voltage of 15 kV; beam current of 50 nA; pixel size of 5 × 5 μm; and counting time of 100 ms. Calcium silicate (CaSiO3) and strontianite (SrTiO3) were used as standards for the analysis of profiles and two-dimensional X-ray intensity maps.
Otolith Sr:Ca ratios are influenced by the Sr content in habitats and food sources (Campana 1999). We utilized Sr:Ca ratio profiles to determine the timing of the first sea entry and discriminate habitat use. First, Sr:Ca ratios outside the 0–10 × 103 range were treated as noise and excluded. Data with isolated spikes in the Sr:Ca ratio or significantly lower Ca abundance compared to surrounding points were also disregarded as likely noise caused by surface irregularities of the otolith or equipment malfunction. To account for the maternal marine experience, we used measurements from points outside the otolith core (<80 μm), as described by Noda et al. (2021). Next, we determined the otolith radius at the first sea entry (hereafter referred to as Ofse) by the initial sharp increase in the Sr:Ca ratio (i.e., >4 × 103), indicating a departure from freshwater, following the method of Arai and Morita (2005). The age of the first sea-run migration was determined based on the annulus closest to Ofse. Finally, we categorized between ocean, brackish, and freshwater periods using the following criteria: (1) Ocean period, characterized by Sr:Ca ratios higher than 4 × 103. (2) Brackish water period, characterized by Sr:Ca ratios with a relatively intermediate increase that was lower than 4 × 103 but higher than that of freshwater resident charr captured in the same river. (3) Freshwater period, characterized by Sr:Ca ratios lower than 4 × 103 or values similar to freshwater resident charr captured in the same river.
Categorizing migratory patterns. To evaluate the migratory diversity of anadromous white-spotted charr, we classified lifetime migration patterns of 193 anadromous migrants using otolith Sr:Ca ratios. First, migratory histories were divided into two stages: (A) the virgin sea-run stage, which refers to the phase prior to the first sea entry (corresponding to the Sr:Ca ratio profiles from core to Ofse), and (B) the veteran sea-run stage, which refers to the phase after the first sea entry (corresponding to the Sr:Ca ratio profiles from Ofse to edge) (Table 1, Fig. 2).
Schematic diagram showing habitat use in a migration patterns at the virgin sea-run stage and b migration patterns at the veteran sea-run stage of anadromous white-spotted charr. Dashed lines and numbers indicate annuli (opaque) and age, respectively. Black arrowheads indicate the first sea entry. Normal stands for sea entry at age ≥1+ years old
Next, the Sr:Ca profiles at the virgin sea-run stage were categorized into three types: (A1) ocean entry at age ≥1+ years old, hereafter denoted as “normal”, (A2) ocean entry at age 0+ years old, hereafter denoted as “early descending”, and (A3) brackish use prior to the first sea entry, hereafter denoted as “brackish use”. As smolting in this species usually occurs at ages older than 2+ years (Yamamoto and Morita 2002), we defined individuals that descended to the sea at age 0+ years as “early descending”.
Additionally, the profiles of the veteran sea-run stage were grouped into four types based on the annual periodicity of ocean and river use: (B1) annual migrants, characterized by periodically fluctuating Sr:Ca ratios aligned with the position of the annuli; (B2) frequent migrants, indicated by multiple peaks in Sr:Ca ratios between annuli; (B3) retired migrants, identified by consistently low Sr:Ca ratios for a certain interval of annuli, the term was originally proposed by Bond et al. (2015) though skipped migration was not included; (B4) ocean residents, marked by consistently high Sr:Ca ratios for a certain interval of annuli.
Estimation of size at first migration. Following Campana (1990), the fork length at first sea entry (Lfse) was estimated using the biological intercept method.
where LT and OT represent the measured FL and otolith radius at capture, respectively, Ofse is the otolith radius at first high Sr:Ca ratios, L0 and O0 are the FL and otolith radius at fry emergence (L0 = 22.8 mm and O0 = 142.6 μm based on digitizing data from figures in Tsukamoto et al. 1989).
Statistics. A generalized linear mixed model (GLMM) was employed to examine the life-history traits of different migratory patterns. FL, age, and CF at capture were analyzed using GLMMs with migratory types of the virgin sea-run stage (fixed effect), migratory types of the veteran sea-run stage (fixed effect), sex (fixed effect), and river (random effect) as predictor variables with a normal distribution of errors. Interaction terms between the virgin and veteran sea-run stages were not considered due to the small sample size of the specific combination. The F-test was used to assess the significance of each predictor variable. Additionally, Turkey-Kramer multiple comparison tests were performed to compare FL and age between the different migratory types.
To compare life-history traits at the first sea-run, a critical period in migration, the estimated FL and age at the first sea-run were also analyzed using GLMMs with migratory patterns of the veteran sea-run stage (fixed effect), sex (fixed effect), and river (random effect) as predictor variables with a normal distribution of errors. Turkey-Kramer multiple comparison tests were subsequently performed. Fisher’s exact test was used to assess the frequency of migratory types among rivers at both the virgin and veteran sea-run stages. Additionally, Fisher’s exact test with Bonferroni corrections was applied to determine whether the frequency of migratory patterns differed across all combinations of river groups after the number of individuals with each migratory pattern was counted for each of the three river groups (northern Hokkaido, southwestern Hokkaido, and Honshu).
All statistical analyses were conducted using R version 4.2.1 (R Development Core Team 2022). The “lme4” package was utilized for running the GLMM.
Results
Lifetime migratory patterns. Patterns of life history, as revealed through transect analysis and two-dimensional X-ray intensity maps of Sr and Ca, showed remarkable variation among individuals (Figs. 3 and 4). The lifetime migratory patterns of anadromous white-spotted charr were classified into ten patterns. These patterns were represented by a combination of three types at the virgin sea-run stage and four types at the veteran sea-run stage (Table 2).
Changes in otolith Sr:Ca ratios from core to edge in white-spotted charr. Grey bands indicate annuli and red lines indicate first sea entry. White, gray, and black bars indicate freshwater rivers, brackish water, and oceanic periods, respectively. A1-B1 Ocean entry at age ≥1+ years old (normal)-annual migrant (4+ age, 270 mm FL), A2-B1 early descending-annual migrant (3+ age, 366 mm FL), A3-B1 brackish use-annual migrant (4+ age, 315 mm FL), A3-B2 brackish use-frequent migrant (3+ age, 354 mm FL), A1-B3 normal-retired migrant (4+ age, 364 mm FL), A1-B4 normal-ocean resident (4+ age, 385 mm FL)
X-ray intensity maps of otolith Sr:Ca ratios in white-spotted charr. The values corresponding to Sr:Ca ratios are represented by 256 colors, from red (highest) to yellow to green to blue (lowest). Scale bar indicates 1 mm. A1-B1 Ocean entry at age ≥1+ years old (normal)-annual migrant (7+ age, 443 mm FL), A2-B1 early descending-annual migrant (4+ age, 325 mm FL), A3-B1 brackish use-annual migrant (4+ age, 315 mm FL), A1-B2 normal-frequent migrant (3+ age, 360 mm FL), A1-B3 normal-retired migrant (4+ age, 364 mm FL), A1-B4 normal-ocean resident (4+ age, 385 mm FL)
Fish classified as normal-annual migrants (A1-B1) were the most common, accounting for 66.8% of the observed lifetime migratory patterns. These fish inhabited freshwater rivers for 1+ to 5+ years and then migrated annually between the river and ocean (A1-B1 in Figs. 3 and 4). The second most abundant pattern was that of brackish use-annual migrants (A3-B1), constituting 14.5% of the sample. These individuals initially utilized brackish areas at least one year before their first sea entry (A3-B1 in Figs. 3 and 4). Normal-retired migrants (A1-B3) were the third most common, making up 5.7% of the individuals. These fish suspended migration and remained in the river for over a year during the veteran sea-run stage (A1-B3 in Figs. 3 and 4). Notably, four of the 15 retired individuals at the veteran sea-run stage exhibited “skipped migration”, migrated to the sea again after a period of migration suspension (ESM Fig. S2). However, it should be noted that the terms “retired” and “skipped” do not mean that they stopped reproduction.
Focusing on the timing of rare migration patterns at the veteran sea-run stage, frequent migrants moved between rivers and the ocean multiple times within a year, across different calendar years or at different ages (A3-B2 in Fig. 3, A1-B2 in Fig. 4). Ocean residents, on the other hand, either stayed in the ocean for an entire year or made only brief migrations to the river (A1-B4 in Figs. 3 and 4). While the age at the time of ocean residence varied (from age 1+ to 8+), the calendar year of ocean residence was synchronized in 2018 among seven of the eight ocean resident individuals.
Composition of migration patterns. The composition of migratory patterns varied among rivers, as indicated in Fig. 5 and ESM Table S3. Multiple migration patterns coexisted within the same river, with a maximum of five patterns observed in both the Moshosanbetsu River in northern Hokkaido and the Ori River in southwestern Hokkaido. The composition of migratory types at the virgin sea-run stage differed significantly between rivers (Fisher’s exact test, p < 0.01). The “normal” migratory type, which spends more than a year in the river during the parr stage before descending to the sea, was the most common, accounting for 77.7% of the sample. “Brackish use” was more frequent at 16.6% than “early descending” which occurred at 5.7% (Table 2). However, the frequency of early descent was notably higher in the Chiyoshibetsu River, characterized by its relatively steep gradient, compared to other rivers (Fig. 5).
The proportions of migratory patterns at the a virgin and b veteran sea-run stages of white-spotted charr in each study river and latitude. N indicates the number of individuals, and the numbers in the component bar chart indicate the percentage of each migratory pattern. Normal stands for Ocean entry at age ≥1+ years old
Significant variation also existed in the proportion of migratory types at the veteran sea-run stage between rivers (Fisher’s exact test, p < 0.001). The frequency of migratory types at the veteran sea-run stage differed significantly between the northern and southwestern Hokkaido River groups (Fisher’s exact test, p < 0.01 after Bonferroni correction) and between the northern Hokkaido and Honshu River groups across the Tsugaru Strait (Fisher’s exact test, p < 0.001 after Bonferroni correction). However, no significant differences were detected between the southwestern Hokkaido and Honshu River groups. A geographical trend emerged: ocean residents were primarily found in the north, while retired migrants were more common in the south (Fig. 5). In the Toji River, the southernmost river in this study, nearly half of the individuals (52.6%) were classified as retired migrants at the veteran sea-run stage.
Biological traits. The mean FLs (± SD) and mean ages (± SD) of the 193 anadromous white-spotted charr were 348.2 ± 75.5 mm (range 209–630 mm) and 3.7 ± 1.5 years (range 2+–9+), respectively. The back-calculated FLs at first sea entry were 219.4 ± 58.7 mm (range 29–331 mm), and the ages at first sea entry were 2.3 ± 0.92 years (range 0+–5+). Notably, some individuals descended to the sea at extremely small sizes (e.g., 29.2 mm).
The GLMM showed that FL at the time of capture differed significantly among migration types at the veteran sea-run stage (F3, 182 = 6.00, p < 0.001). However, FL did not differ based on migration types at the virgin sea-run stage (F2, 182 = 0.46, p = 0.62) or according to sex (F1, 177 = 1.81, p = 0.18). Annual migrants were significantly smaller than frequent and retired migrants (Tukey-Kramer test, p < 0.05) (Fig. 6a). No significant differences were observed in the mean FL among the other types (p = 0.51–0.99).
Comparison of 193 white-spotted charr’s a fork length (FL, mm) at capture, b age at capture (year), c estimated fork length at first sea entry (mm), and d estimated age at first sea entry (year) between four migratory patterns at the veteran sea-run stage. Central lines show medians, boxes show the interquartile range (central 50% of data points), whiskers bracket 1.5 times the interquartile range, solid circles identify outliers, and open circles show means. Asterisks indicate significant differences (*p < 0.05, **p < 0.01, ***p < 0.001)
Migration patterns at both the virgin and veteran sea-run stages exhibited significant influences on age at capture (virgin sea-run stage: F2, 178 = 3.23, p < 0.05; veteran sea-run stage: F3, 178 = 9.50, p < 0.001). However, age at capture did not differ according to sex (F1, 174 = 0.02, p = 0.88). Multiple comparisons revealed that annual migrants were significantly younger than retired migrants (Tukey-Kramer test, p < 0.001) (Fig. 6b). No significant differences of age at capture were noted among the migratory types at the virgin sea-run stage (Tukey-Kramer test: normal vs. brackish use, p = 0.36; normal vs. early descending, p = 0.051; brackish use vs. early descending, p = 0.31).
CFs at the time of capture differed significantly among migration types at the virgin sea-run stage (F2, 184 = 3.20, p < 0.05) and were larger for females (F1, 183 = 19.7, p < 0.001). However, the CF did not differ based on migration types at the veteran sea-run stage (F3, 179 = 0.65, p = 0.58). Early descending showed a larger CF than those of normal migrants (Tukey-Kramer test, p < 0.05) and brackish type (Tukey-Kramer test, p = 0.07).
FLs at the first sea entry differed significantly among migration types at the veteran sea-run stage (F3, 186 = 10.1, p < 0.001) and were larger for females (F1, 180 = 5.01, p < 0.05). Multiple comparisons indicated that retired migrants entered the sea initially with significantly smaller FLs than the other migratory types (Tukey-Kramer test, p < 0.01) (Fig. 6c). Additionally, the age at first sea entry also varied significantly among migration patterns at the veteran sea-run stage (F3, 186 = 10.4, p < 0.001), but did not differ by sex (F1, 181 = 0.0089, p = 0.93). Retired migrants at first sea entry were significantly younger than other migratory types (Tukey-Kramer test, p < 0.01) (Fig. 6d).
Discussion
White-spotted charr exhibited at least 10 different lifetime migration patterns in rivers flowing into the Sea of Japan. While the majority of individuals adhered to a well-known migratory pattern (i.e., normal-annual migrants), several rare migration patterns (i.e., retired migrants and ocean residents) emerged that have not been previously reported (Fig. 7). Thus, the migration patterns of white-spotted charr are more flexible and complex than previously described. Additionally, each migration pattern featured different timings and periods for migration, and the degree of oceanic dependence varied among the patterns. Overall, the migration patterns of white-spotted charr were more ocean-dependent in northern rivers, although some specific migratory patterns may reflect local adaptations.
Migratory patterns. Migration patterns during the virgin sea-run stage were categorized into three types: normal, early descending, and brackish use. “Normal” was the most abundant migratory pattern observed in this study. In general, white-spotted charr parr transformed into smolts and migrated to the sea at ages ranging from 2+ to 5+ years (Yamamoto and Nakano 1996; Yamamoto and Morita 2002). The otolith Sr:Ca ratios of normal individuals confirmed that sea-run migration occurred between the ages of 1+ and 5+ years, which is mostly consistent with previous studies. In this study, ocean entry at age 1+ was primarily identified in rivers of the south, specifically in southwestern Hokkaido and Honshu. However, it is reasonable to consider that smolt age varies with juvenile growth rate, with older smolts being found at higher latitudes (Yamamoto and Morita 2002).
Some individuals exhibiting consistently high otolith Sr:Ca ratios throughout their lives have been reported as “estuarine residents” in the previous study (Arai and Morita 2005; Table 3), corresponding to early descending and brackish use types in the present study. One possible explanation for the variation in the occurrence of early descending may be the degree of the river gradient. Several previous otolith microchemistry studies on sympatric salmonid species have also identified pre-smolt migrants from steep-gradient rivers (e.g., masu salmon Oncorhynchus masou, Kuroki et al. 2020; southern Asian Dolly Varden Salvelinus curilus, Umatani et al. 2018; Table 3). Due to their limited swimming capability, juvenile fish are likely to be displaced downstream (Lechner et al. 2016). White-spotted charr with less sedentary behavior has been shown to cause downstream displacement (Yamada and Wada 2023). Furthermore, juvenile white-spotted charr exhibits seawater adaptability without acclimation (Gorie 1996; Takami 1998). Therefore, passively downstream-displaced white-spotted char may be able to survive in the sea even at the parr stage and return to rivers.
Some salmonid species, like the brown trout (Limburg et al. 2001; Wynne et al. 2023), coho salmon Oncorhynchus kisutch (Koski 2009; Jones et al. 2014, 2021), and Chinook salmon Oncorhynchus tshawytscha (Chalde and Fernández 2017), utilize estuarine areas at the parr stage. Estuaries provide highly productive environments that enable juvenile salmon to grow larger than those in freshwater rivers (Quinn 2018). As indicated by experimental studies exposing salmonid fish to varying salinity levels (Teskeredžić et al. 1989; Mardones et al. 2020), brackish water potentially enhances the growth of charr by reducing osmoregulation cost. Moreover, juvenile rainbow trout Oncorhynchus mykiss exposed to temporary seawater achieve higher growth and seawater acclimability in later life stages (Kaneko et al. 2019). Although there were no differences in FL or age at the first sea-run, early descending showed a significantly higher CF at capture than normal in this study, such that early descending or brackish white-spotted charr, which appears in a certain proportion of each river, may actively migrate to estuaries and benefit in some aspects of subsequent growth and survival.
During the veteran sea-run stage, the migratory patterns of the white-spotted char were classified into four types: annual migrants, frequent migrants, retired migrants, and ocean residents. Annual migrants were the most common migratory type in this study, aligning with well-known migration patterns reported in previous studies (e.g., Takami 1995; Takami et al. 1996; Sahashi and Morita 2024). These individuals migrate to the sea at approximately ages 2+ to 5+ years and repeat their annual oceanic migration. Frequent migration patterns were consistent with those suggested by the bio-logging method or otolith marking with tag-recaptured data, with some individuals reported to exhibit frequent short-term migrations (daily to monthly) between fresh and saltwater environments (Arai and Morita 2005; Morita et al. 2013; Kuroda and Miyashita 2022). The iteroparous Salvelinus individuals spawn upstream in their natal river, but are not limited to the natal river when migrating upstream for purposes other than spawning (Armstrong and Morrow 1980; Armstrong 1984; DeCicco 1997). That is, the purposes of migration between the coastal area and the natal or surrounding rivers include overwintering, summering, and feeding. (Takami 1995; Morita et al. 2013; Kuroda and Miyashita 2022). The habitat use of genus Salvelinus include white-spotted charr appears to be more flexible than the spatially and temporally extensive migration of salmonid species (Quinn 2021). However, otolith microchemical composition does not provide daily resolution because the otolith Sr:Ca ratio response to ambient water has a time lag due to species, physiology, and life-history stages (Kalish 1989; Campana 1999). Considering that the measurement of spatial resolution corresponded to approximately 40 days in old individuals, the detection of such short-term migration using otolith microchemistry may be challenging. This limitation may have led to an underestimation of the number of frequent migrants.
The migration patterns of ocean residents and retired migrants have been first reported for white-spotted charr by this study. The occurrence of these patterns exhibited geographical trends; ocean residents appeared in the northern regions, but retired migrants appeared in the southern rivers, indicating that ocean-dependent migration patterns are more common at high latitudes. Similar migration patterns have been observed in several species of the genus Salvelinus (Table 3). Austin et al. (2019) demonstrated that the bull trout Salvelinus confluentus exhibited a “skipped migration”, undergoing either a prolonged ocean migration or skipping seaward migration for a year, corresponding to retired migrants and ocean residents in our study. Bond et al. (2015) found that the older Dolly Varden Salvelinus malma ceased migration.
The timing of the cessation or resumption of migration was not synchronized among individuals by age, suggesting that the decision may be facultative. Seawater temperature is inferred to be a direct factor limiting ocean migratory behavior. Anadromous Arctic charr and Dolly Varden inhabiting colder regions obligatorily overwinter in the freshwater (Bond and Quinn 2013; Smith et al. 2022) due to a decrease in hypo-osmoregulatory capacity with photoperiodic changes under low water temperatures (Finstad et al. 1989). Takami (1995) pointed out that the overwintering of white-spotted charr in the sea occurs only in southern regions due to relatively higher winter water temperatures. The seawater tolerance of white-spotted charr is affected by water temperature, with osmoregulatory capacity declining at 16 °C and seawater tolerance significantly reducing at temperatures above 20 °C (Takami 1998). In the Sea of Japan, dominated by the Tsushima Warm Current, maximum sea surface temperature (SST) is spatially lower (approximately 20 °C) at higher latitudes and higher (approximately 27 °C) at lower latitudes. Additionally, the period of mean SST above 20 °C is 130 days in a year at the southernmost habitat (38°N) (JMA 2021). In the summer of 2018, the SST was locally 2 °C lower than the annual average, which explains why seven of the eight ocean resident individuals were able to spend the summer in the northern ocean. In addition, the migration of white-spotted charr generally occurs along the coast and at relatively short distances from the natal river mouth (Takami 1995; Aoyama 1997). Consequently, white-spotted charr are unable to overwinter in the extremely cold northern habitats, and cannot oversummer in the ocean in southern habitats, where thermal refuges are not available. In other words, ocean residents appear only in thermally optimal habitats.
Contrary to ocean residence being limited by seawater temperature, the appearance of retired migrants may be due to the relatively less risky and resource-rich environment than the ocean in southern rivers, rather than solely water temperature. Ocean productivity is expected to be higher than that in the surrounding freshwater environments at high latitudes (Gross et al. 1988). Salmonids, which are widely distributed at high latitudes, are typically anadromous, defined as fish that spend most of their lives in the ocean and migrate to freshwater to breed (McDowall 1992). In other words, they rely on the ocean for growth. This also holds true for anadromous white-spotted charr, as they actively feed on small pelagic fishes in the ocean but fast in rivers in their northern distribution (Takami et al. 1996; Savvaitova et al. 2007). However, in southern habitats, where high water temperatures limit the duration of ocean migration, white-spotted charr may store only a minimal amount of energy for migration. Dolly Varden, in rivers with abundant spawning salmon, can obtain sufficient energy by consuming salmon eggs, providing residents with an advantage and rendering migration unnecessary (Armstrong and Bond 2013). In other words, if rivers provide sufficient food, charr need not migrate to the ocean, which exposes them to risk. Southern anadromous white-spotted charr actively feeds on fallen insects in rivers, and their body condition is similar to that of the northern charr (Goto et al. 2023). Therefore, it is inferred that retired migrants are more likely to appear at the southern limit of their distribution because the rivers in this region provide an environment where they can obtain sufficient energy.
Biological traits of each migratory category. The larger size and older age of individuals in the catch of retired migrants compared to that of annual migrants can be attributed to various factors. The evidence is limited to a comprehensive evaluation; however, a few plausible explanations are suggested. A simple explanation for this could be detection bias. It takes several years to classify each migratory pattern after a virgin sea-run. Consequently, younger individuals are more likely to be classified as annual migrants, whereas older individuals tend to be classified as having rare migratory patterns (i.e., retired migrants). The second aspect is the balance between the benefits and costs of oceanic migration. In Dolly Varden in the Alec River, Alaska (56°N), retired migrants were dominant (92.7%), with older individuals retired from anadromy (100% at age 9, 85.7% at age 8, 89.5% at age 7 years) (Bond et al. 2015). The study proposed that once an individual reaches near-maximum size, the fitness benefits of continued migration may be outweighed by the mortality risk in the marine environment. While this may certainly be the case for retired migrants in the present study, it does not fully explain the resumption of migration as it differs from Bond et al. (2015) in that skipped migration was also detected. In addition, differences in the spawning frequency for each migration pattern may be associated with longevity. Gallagher et al. (2018) examined whether spawning frequency differed between skipped and annual migrants and found that individuals who periodically skipped an annual oceanic migration exhibited skipped spawning and had higher longevity than annual migrants. Thus, different migration tactics result in trade-offs between spawning frequency and longevity. Although Gudkov (1992) noted that some individuals skipped the second reproductive season in northern populations of white-spotted charr, specific information regarding the relationship between migration patterns and reproductive frequency is lacking. Further studies on the relationship between migration patterns and reproductive frequency would provide more insight into the discussion of energy budgets and lifetime fitness.
The alternative life histories of salmonids are explained by a status-dependent conditional strategy (Gross 1996), in which favorable early growth often promotes residency. This strategy has also been observed in white-spotted charr, where poor growing and inferior individuals in rivers become migrants (Yamamoto et al. 1996; Morita et al. 2000; Pichugin et al. 2006). The survival of white-spotted charr in the sea is ultimately determined by size rather than age, with smaller smolts having a survival disadvantage (Yamamoto et al. 1999b). The present study demonstrated that retired migrants migrate to the sea at a younger age and smaller size, indicating a higher mortality risk than other migrants. Oceans are more productive than freshwater environments but also entail higher risks (Jonsson and Jonsson 1993; Gross et al. 1988). Therefore, retired migrants may derive significant benefits from early migration if they survive. Since the present study used back-calculated data from up-migrating individuals that survived in the ocean, further research is needed to address the survival bias.
Implications of migratory diversity. It is noteworthy that multiple migration patterns co-appeared in the small river in this study. These individual migration patterns may bolster the resilience of ecosystem services. For threatened Chinook salmon populations on the southern edge of their habitat in California, rare migratory phenotypes—characterized by variations in age and size at the time of migration from their natal tributary—contribute to the long-term persistence of the population (Cordoleani et al. 2021). In the Salmon River, Oregon, Chinook salmon have exhibited greater early life history variation following the removal of estuarine levees, which enables them to utilize a broader spectrum of estuarine habitats (Bottom et al. 2005). Highlighting the significance of habitats along the fresh-to-saltwater continuum and broadening habitat opportunities for salmon populations to express their full life history variation is pivotal in fortifying ecosystem resilience (Flitcroft et al. 2016). Preserving the diverse life histories of salmon enhances socio-ecological resilience by offering ecosystem services, including consistent stream flows, clean water, functional wetlands and floodplains, and thriving fisheries (Bottom et al. 2009).
References
Aoyama T (1997) Long distance movements of anadromous white-spotted charr (Salvelinus leucomaenis) in southern Hokkaido, Japan. Sci Rep Hokkaido Fish Hatch 51:63–65
Arai T, Morita K (2005) Evidence of multiple migrations between freshwater and marine habitats of Salvelinus leucomaenis. J Fish Biol 66:888–895
Armstrong RH (1984) Migration of anadromous Dolly Varden charr in southeastern Alaska―A manager’s nightmare. In: Johnson L, Burns B (eds) Biology of the Arctic charr: Proceedings of the International Symposium on Arctic Charr. University of Manitoba Press, Winnipeg, pp 559–570
Armstrong JB, Bond MH (2013) Phenotype flexibility in wild fish: Dolly Varden regulate assimilative capacity to capitalize on annual pulsed subsidies. J Anim Ecol 82:966–975
Armstrong RH, Morrow JE (1980) The dolly varden charr, Salvelinus malma. In: Balon EK (ed) Charrs: salmonid fishes of the genus Salvelinus. Dr. W. Junk Publishers, the Hague, pp 99–140
Austin CS, Bond MH, Smith JM, Lowery ED, Quinn TP (2019) Otolith microchemistry reveals partial migration and life history variation in a facultatively anadromous, iteroparous salmonid, bull trout (Salvelinus confluentus). Environ Biol Fishes 102:95–104
Bond MH, Miller JA, Quinn TP, Ciannelli L (2015) Beyond dichotomous life histories in partially migrating populations: cessation of anadromy in a long-lived fish. Ecology 96:1899–1910
Bond MH, Quinn TP (2013) Patterns and influences on Dolly Varden migratory timing in the Chignik Lakes, Alaska, and comparison of populations throughout the northeastern Pacific and Arctic oceans. Can J Fish Aquat Sci 70:655–665
Bottom DL, Jones KK, Cornwell TJ, Gray A, Simenstad CA (2005) Patterns of Chinook salmon migration and residency in the Salmon River estuary (Oregon). Estuar Coast Shelf Sci 64:79–93
Bottom DL, Jones KK, Simenstad CA, Smith CL (2009) Reconnecting social and ecological resilience in salmon ecosystems. Ecol Soc 14:5
Brenkman SJ, Corbett SC, Volk EC (2007) Use of otolith chemistry and radiotelemetry to determine age-specific migratory patterns of anadromous bull trout in the Hoh River, Washington. Trans Am Fish Soc 136:1–11
Campana SE (1990) How reliable are growth back-calculations based on otoliths? Can J Fish Aquat Sci 47:2219–2227
Campana SE (1999) Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser 188:263–297
Caputo M (2013) Migratory behaviour of brook charr (Salvelinus fontinalis) in Gros Morne National Park, Newfoundland, and the potential for co-management of the recreational fishery. Master Dissertation, Memorial University of Newfoundland
Chalde T, Fernández DA (2017) Early migration and estuary stopover of introduced chinook salmon population in the Lapataia River Basin, southern Tierra del Fuego Island. Estuar Coast Shelf Sci 199:49–58
Chin AT, Bond MH, Quinn TP (2022) Life history differences between 2 anadromous populations of the northern form of the Dolly Varden (Salvelinus malma malma) in Bristol Bay in southwestern Alaska. Fish Bull 120:234–239
Cordoleani F, Phillis CC, Sturrock AM, FitzGerald AM, Malkassian A, Whitman GE, Weber PK, Johnson RC (2021) Threatened salmon rely on a rare life history strategy in a warming landscape. Nat Clim Chang 11:982–988
Cucherousset J, Ombredane D, Charles K, Marchand F, Baglinière JL (2005) A continuum of life history tactics in a brown trout (Salmo trutta) population. Can J Fish Aquat Sci 62:1600–1610
DeCicco AL (1997) Movements of postsmolt anadromous Dolly Varden in Northwestern Alaska. Am Fish Soc Symp 19:175–183
Finstad B, Nilssen KJ, Arnesen AM (1989) Seasonal changes in sea-water tolerance of Arctic charr (Salvelinus alpinus). J Comp Physiol B 159:371–378
Flitcroft RL, Bottom DL, Haberman KL, Bierly KF, Jones KK, Simenstad CA, Gray A, Ellingson KS, Baumgartner E, Cornwell TJ, Campbell LA (2016) Expect the unexpected: place-based protections can lead to unforeseen benefits. Aquat Conserv 26:39–59
Gallagher CP, Howland KL, Sandstrom SJ, Halden NM (2018) Migration tactics affect spawning frequency in an iteroparous salmonid (Salvelinus malma) from the Arctic. PLoS One 13:e0210202
Gorie S (1996) Evaluation of seawater adaptability of 0+ Japanese charr related to possibility of the culture in seawater. Fish Sci 62:384–387
Goto A, Kuroki M, Morita K (2023) Active feeding of anadromous white-spotted charr Salvelinus leucomaenis at the southern latitudinal rivers. Can J Fish Aquat Sci 80:1737–1747
Gross MR (1996) Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol 2:92–98
Gross MR, Coleman RM, McDowall RM (1988) Aquatic productivity and the evolution of diadromous fish migration. Science 239:1291–1293
Gudkov PK (1992) Data on the biology of the far eastern char, Salvelinus leucomaenis, in the sea of Okhotsk Basin. J Ichthyol 32:9–23
Japan Meteorological Agency (2021) Sea surface temperature information for coastal areas of Japan https://www.data.jma.go.jp/kaiyou/data/db/kaikyo/series/engan/engan.html Accessed 24 December 2021
Jensen JLA, Rikardsen AH (2012) Archival tags reveal that Arctic charr Salvelinus alpinus and brown trout Salmo trutta can use estuarine and marine waters during winter. J Fish Biol 81:735–749
Jones KK, Cornwell TJ, Bottom DL, Campbell LA, Stein S (2014) The contribution of estuary-resident life histories to the return of adult Oncorhynchus kisutch. J Fish Biol 85:52–80
Jones KK, Cornwell TJ, Bottom DL, Stein S, Starcevich S (2021) Interannual variability in life-stage specific survival and life history diversity of coho salmon in a coastal Oregon basin. Can J Fish Aquat Sci 78:1887–1899
Jonsson B, Jonsson N (1993) Partial migration: niche shift versus sexual maturation in fishes. Rev Fish Biol Fish 3:348–365
Jonsson IR, Antonsson T (2005) Emigration of age-1 Arctic charr, Salvelinus alpinus, into a brackish lagoon. Environ Biol Fishes 74:195–200
Kalish JM (1989) Otolith microchemistry: validation of the effects of physiology, age and environment on otolith composition. J Exp Mar Biol Ecol 132:151–178
Kaneko T, Suzuki R, Watanabe S, Miyanishi H, Matsuzawa S, Furihata M, Ishida N (2019) Past seawater experience enhances subsequent growth and seawater acclimability in a later life stage in rainbow trout Oncorhynchus mykiss. Fish Sci 85:925–930
Kissinger BC, Gantner N, Anderson WG, Gillis DM, Halden NM, Harwood LA, Reist JD (2016) Brackish-water residency and semi-anadromy in Arctic lake trout (Salvelinus namaycush) inferred from otolith microchemistry. J Great Lakes Res 42:267–275
Klemetsen A (2013) The most variable vertebrate on Earth. J Ichthyol 53:781–791
Koski KV (2009) The fate of coho salmon nomads: the story of an estuarine-rearing. Ecol Soc 14:4
Kuroda M, Miyashita K (2022) Winter migratory pattern for anadromous white-spotted char (Salvelinus leucomaenis) in southwestern Hokkaido, Japan. Environ Biol Fishes 105:1845–1855
Kuroki M, Tamate T, Morita K (2020) An additional life-history tactic of masu salmon: migration of parr to coastal habitats. Ecol Freshw Fish 29:495–501
Lechner A, Keckeis H, Humphries P (2016) Patterns and processes in the drift of early developmental stages of fish in rivers: a review. Rev Fish Biol Fish 26:471–489
Limburg KE, Landergren P, Westin L, Elfman M, Kristiansson P (2001) Flexible modes of anadromy in Baltic sea trout: making the most of marginal spawning streams. J Fish Biol 59:682–695
Maekawa K, Nakano S (2002) To sea or not to sea: a brief review on salmon migration evolution. Fish Sci 68:27–32
Mainguy J, Arsenault A, Tran L, Martyniuk MAC, Paquet C, Moore J, Power M (2023) Otolith‐inferred patterns of marine migration frequency in Nunavik Arctic charr. J Fish Biol 103:884–896
Mardones A, Vega R, Encina F, Pichara C, González K, De los Rios P, Peña B (2020) Assessing the growth of Arctic charr (Salvelinus alpinus) (Linnaeus, 1758) in four salinities, under experimental conditions. Braz J Biol 80:907–913
McDowall RM (1992) Diadromy: origins and definitions of terminology. Copeia 1992:248–251
Morita K, Morita S, Nagasawa T, Kuroki M (2013) Migratory patterns of anadromous white-spotted charr Salvelinus leucomaenis in Eastern Hokkaido, Japan: the solution to a mystery? J Ichthyol 53:809–819
Morita K, Yamamoto S, Hoshino N (2000) Extreme life history change of white-spotted char (Salvelinus leucomaenis) after damming. Can J Fish Aquat Sci 57:1300–1306
Noda S, Ueda R, Tanaka T, Shirai K, Kishi D, Sato T (2021) Anadromous red-spotted masu salmon (Oncorhynchus masou ishikawae), a southernmost sea-migration form of salmonid, displays low variation in both age at seaward migration and sea age. J Fish Biol 99:1497–1502
Pichugin MY, Sidorov LK, Gritsenko OF (2006) New data on the trout Salvelinus leucomaenis and its relationships with Dolly Varden trout S. malma curilis in fresh waters of the southern Kuril Islands. J Ichthyol 46:369–382
Price MHH, Moore JW, Connors BM, Wilson KL, Reynolds JD (2021) Portfolio simplification arising from a century of change in salmon population diversity and artificial production. J Appl Ecol 5:1477–1486
Quinn TP (2018) The behavior and ecology of Pacific salmon and trout: second edition. University of British Columbia Press, Vancouver
Quinn TP (2021) Differential migration in Pacific salmon and trout: patterns and hypotheses. Anim Migr 8:1–18
Quinn TP, Myers KW (2004) Anadromy and the marine migrations of Pacific salmon and trout: Rounsefell revisited. Rev Fish Biol Fish 14:421–442
R Development Core Team (2022) R: a language and environment for statistical computing. R foundation for statistical Computing, Vienna
Radtke R, Svenning M, Malone D, Klementsen A, Ruzicka J, Fey D (1996) Migrations in an extreme northern population of Arctic charr Salvelinus alpinus: insights from otolith microchemistry. Mar Ecol Prog Ser 136:13–23
Roloson SD, Knysh KM, Landsman SJ, James TL, Hicks BJ, van den Heuvel MR (2022) The lifetime migratory history of anadromous brook trout (Salvelinus fontinalis): insights and risks from pesticide-induced fish kills. Fishes 7:109
Rounsefell GA (1958) North American Salmonidae. Fish Bull 58:171–185
Sahashi G, Morita K (2024) Partial migration in salmonids: focusing on Asian endemic masu salmon (Oncorhynchus masou) and white-spotted charr (Salvelinus leucomaenis). In: Lobon-Cervia J, Budy P, Gresswell R (eds) Advances in the population ecology of stream salmonids. Springer Nature, Germany
Savvaitova KA, Kuzishchin KV, Pichugin MY, Gruzdeva MA, Pavlov DS (2007) Systematics and biology of the East Siberian char Salvelinus leucomaenis. J Ichthyol 47:53–66
Smith R, Hitkolok E, Loewen T, Dumond A, Kristensen K, Swanson H (2022) Overwinter ecology and movement of anadromous Arctic char (Salvelinus alpinus) in a large, ice-covered river in the Canadian Arctic. J Fish Biol 100:1432–1446
Spares AD, Dadswell MJ, Dickinson MP, Stokesbury MJW (2015) A critical review of marine adaptability within the anadromous Salmoninae. Rev Fish Biol Fish 25:503–519
Swanson HK, Kidd KA, Babaluk JA, Wastle RJ, Yang PP, Halden NM, Reist JD (2010) Anadromy in Arctic populations of lake trout (Salvelinus namaycush): otolith microchemistry, stable isotopes, and comparisons with Arctic char (Salvelinus alpinus). Can J Fish Aquat Sci 67:842–853
Takami T (1995) Migration of anadromous white-spotted charr, Salvelinus leucomaenis, in southwestern Hokkaido, Japan. Nord J Freshw Res 71:432–437
Takami T (1998) Seawater tolerance of white-spotted charr (Salvelinus leucomaenis) related to water temperature. Sci Rep Hokkaido Fish Hatch 52:11–19
Takami T, Murakami Y, Mori M (1996) Growth and feeding habits of anadromous white-spotted charr (Salvelinus Leucomaenis) in southwestern Hokkaido, Japan. Sci Rep Hokkaido Fish Hatch 50:37–44
Teskeredžić E, Teskeredžić Z, Tomec M, Modrušan Z (1989) A comparison of the growth performance of rainbow trout (Salmo gairdneri) in fresh and brackish water in Yugoslavia. Aquaculture 77:1–10
Tsukamoto K, Seki Y, Oba T, Oya M, Iwahashi M (1989) Application of otolith to migration study of salmonids. Physiol Ecol Japan Spec 1:119–140
Umatani Y, Arai T, Maekawa K (2008) Variation in migratory history of Dolly Varden in a stream with an artificial dam in the Shiretoko Peninsula, Hokkaido, Japan. Environ Biol Fishes 83:37–44
Umatani Y, Arai T, Maekawa K (2018) Flexible seaward migration of Dolly Varden Salvelinus malma in the Shiretoko Peninsula, Hokkaido, Japan. Ichthyol Res 65:202–209
Wynne R, Kaufmann J, Coughlan J, Philips KP, Waters C, Finlay RW, Rogan G, Poole R, McGinnity P, Reed TE (2023) Autumn outmigrants in brown trout (Salmo trutta) are not a demographic dead‐end. J Fish Biol 102:1327–1339
Yamada H, Wada S (2023) Interpopulation variation of behavioural and morphological traits that affect downstream displacement of the juvenile white‐spotted charr Salvelinus leucomaenis. J Fish Biol 102:1168–1176
Yamamoto S, Morita K (2002) Interpopulation comparison of size and age at smolting of white-spotted charr, Salvelinus leucomaenis. Ecol Freshw Fish 11:281–284
Yamamoto S, Morita K, Goto A (1999a) Geographic variations in life-history characteristics of white-spotted charr (Salvelinus leucomaenis). Can J Zool 77:871–878
Yamamoto S, Morita K, Goto A (1999b) Marine growth and survival of white-spotted charr, Salvelinus leucomaenis, in relation to smolt size. Ichthyol Res 46:85–92
Yamamoto S, Morita K, Sahashi G, Maekawa K, Oleinik A, Bondar E, Brykov V (2021) Introgressive hybridization between southern Asian Dolly Varden, Salvelinus curilus, and northern Dolly Varden, S. malma malma, on Sakhalin Island. Russ J Genet 57:361–370
Yamamoto S, Nakano S (1996) Growth and development of a bimodal length-frequency distribution during smolting in a wild population of white-spotted charr in northern Japan. J Fish Biol 48:68–79
Yamamoto S, Nakano S, Tokuda Y (1992) Variation and divergence of the life-history of Japanese charr Salvelinus leucomaenis in an artificial lake-inlet stream system. Japan J Ecol 42:149–157 (in Japanese with English abstract)
Yamamoto S, Takahashi Y, Kitano S, Goto A (1996) Residual female parr in an anadromous population of white-spotted charr, Salvelinus leucomaenis, in southern Hokkaido, Japan. Japan J Ichthyol 43:101–104 (in Japanese)
Zimmerman MS, Sloat MR, Kuzishchin KV, Arostegui MC, Gruzdeva MA, Seamons TR, Quinn TP (2022) Diversity of life history traits, growth, and lipid storage in migratory variants of steelhead and rainbow trout (Oncorhynchus mykiss) in Kamchatka, Russia. Can J Fish Aquat Sci 79:1625–1640
Acknowledgments
The authors thank Ryo Futamura for help with fieldwork and providing samples, and Yoko Kusaba for assistance with the otolith microchemistry analysis. We thank Takashi Yamakawa and Akinori Takasuka for their comments on this study. This study was partially supported by JST SPRING (grant number: JPMJSP2108) and the cooperative program (No. 109, 2022 and No. 111, 2023) of the Atmosphere and Ocean Research Institute, the University of Tokyo.
Funding
Open Access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethics approval
The survey was conducted with the permission of the Governor of Hokkaido, Aomori and Niigata, Japan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Goto, A., Kuroki, M., Shirai, K. et al. Diverse migration patterns of anadromous white-spotted charr Salvelinus leucomaenis revealed from otolith microchemistry. Ichthyol Res (2024). https://doi.org/10.1007/s10228-023-00943-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10228-023-00943-z