Abstract
Behavioural observations were used to investigate the temporal dynamics events and associated variation in the daily activities of a sedentary population of mallard duck (Anas platyrhynchos) inhabiting an urban park in northern Portugal. Data was collected at Porto City Park from December to April, during the species’ courtship and early nesting periods. The most frequent activities for this population were locomotion, resting and feeding. Statistical analysis revealed that male and female behaviours differed significantly throughout the courtship period, as males exhibited more displays and aggressive behaviour while females were more prone to feed in order to meet their increased nutritional and energetic needs. Temporal analyses showed that courtship reached a peak in intensity in February, since it was in this month that the highest values for mating, aggressive behaviour in males and defensive behaviour in females were observed. Behavioural differences were found for this population, with higher feeding rates compared to those of wild populations as well as lower defensive behaviour rates. This paper sheds light on how synanthropized populations, as well as those from Southwestern Europe, show different behavioural responses from other populations. Additional extensive monitoring of population dynamics and factors such as latitudinal differences, habituation to humans and climate change will help to predict future growth and expansion rates.
Similar content being viewed by others
Introduction
In the face of human development, more and more animals have been reported to adapt to manmade conditions and environments. This phenomenon has been observed in many species, mainly those who show ecological plasticity and generalized diet and habitat requirements (review in Luniak 2004). Animals that inhabit anthropized environments tend to undergo several behavioural, morphological and ecological adaptations compared to non-urban populations, including habituation to human presence (Morelli et al 2018), changes in foraging habits (Fernández-Juricic and Telleria 2000), and earlier and more prolonged breeding seasons (Lowry et al. 2013; Raitasuo 1964, in Engel et al. 1988).
One such species that has adapted to urbanized environments is the mallard duck (Anas platyrhynchos L., henceforth “mallard”). This species of waterfowl can adapt to several kinds of wetlands, including manmade landscapes, and is highly tolerant of human activity. Its generalist omnivorous diet (Cramp and Simmons 1977) enables it to procure nourishment other than its natural food sources, and in urban parks it is very common for ducks to be fed by visitors. A substantial body of literature has focused on the behavioural adaptations of Anseriformes to urbanization, captivity and other anthropized conditions in recent years. Donaldson et al. (2007) observed less frequent fleeing behaviours in waterbirds from developed sites in Canada, which can be attributed to habituation to human presence and disturbance. A similar effect was observed in Florida, where ducks exhibited lower alert and fleeing behaviour in developed shorelines (Traut and Hostetler 2003). Avilova et al. (2023) also found that city mallards in Moscow had much shorter flight initiation distances and preferred distances to humans than wild mallards, and often approached people on their own initiative. Downs et al. (2017) showed how domesticated Muscovy ducks (Cairina moschata) were adapted to human activity by observing their differences in habitat use and nesting habits. Rose et al. (2022) noted reduced foraging as well as increased resting for free-ranging captive ducks in the UK compared to wild populations, a probable consequence of higher food availability. In another study (Munday and Rose 2022), captive Mandarin ducks (Aix galericulata) were similarly observed with increased resting behaviour, but also foraged more often than wild individuals.
Similar behavioural studies for the species have not yet been conducted in Portugal, where the mallard is known to display certain ecological adaptations, including establishing mainly resident populations (Rodrigues et al. 2000), as is the case in Spain (Cramp and Simmons 1977; Saez-Royuela and Martinez 1985). There are two distinct regional populations of mallard in Portugal, one residing in the North and Central Regions and one residing in the South; rather than being closely related to each other, they were found to be more closely related to populations from northern Spain and Atlantic Europe and populations living in the south of Spain and the Mediterranean Basin respectively (Rodrigues 2001). Other than these resident breeding populations, some migratory individuals are known to winter in the Iberian Peninsula before moving on to Northern Europe (Rodrigues et al. 2000; Liordos 2010; Parejo et al. 2015). The species’ nesting season in Portugal roughly lasts from early March to July (Cramp and Simmons 1977; Rodrigues 2001). Several ecological studies have been conducted for mallard populations in Portugal (Carvalho et al. 2013; Costa and Guedes 1994; Cruz et al. 2021; Rodrigues et al. 2006; Rufino 1989); however, apart from populations living in rice fields in the Mondego region (Rodrigues et al. 2002; Rodrigues and Fabião 1997), little else is known about the species’ ecology and behaviour in anthropized environments in the country.
Our current understanding of how mallards cope with these environmental conditions and how this leads to differences in the progression of their reproductive cycle is limited, and shedding light on these factors was the main focus of this study. We used land-based observations in an urban park in in the country’s Northern Region to (1) investigate the behavioural activity of a population of mallards residing in the area during the courtship period, (2) analyse the temporal dynamics of these events, (3) assess the variation of activity on land, water and air, social interactions and feeding habits. Considering the species’ ecological characteristics in the country, we predict: 1) this population follows the species’ phenology as described for other populations in the country. Based on the current literature that shows differences in time budgets and defensive behaviours observed for this species in urban environments, we also predict: 2) males and females feature apparent sex differences in their time budgets due to having different roles during courtship; 3) the population exhibits less frequent defensive behaviours as a consequence of habituation to humans.
Methods
Study area
Field research was conducted in Porto City Park, located in Porto, Portugal (41.167º N, 8.674º W). The city, located on the northern coast of Portugal, is characterized by an Atlantic climate with mild wet winters and warm dry summers. Porto City Park has an area of 90 hectares, 80 of which being green areas, including grassland, tree and shrub cover, and four major lakes that are fed by rainfall as well as local springs and watercourses. The free-living mallard population was found at the easternmost lake, known as Lake 1. It is the largest lake in the park, having a surface level of 16,190 m2, a volume of 42,095 m3, and an average depth of 260 cm (data provided by Porto City Hall – Urban Parks Municipal Division). In the shores of the lake, one can find a great variety of riparian vegetation, mainly consisting of common rush (Juncus effusus) and broadleaf cattail (Typha latifolia), that is preserved during maintenance operations, providing shelter to the local fauna. The mallards around these shores were the focus for data collection (Fig. 1). The surrounding area is characterized by wooded expanses and dirt roads that are almost constantly traversed by people during the day, which ducks tend to avoid.
Due to the abundance of food and shelter available, as well as being located near the shoreline, Porto City Park and its lakes can support a wide variety of bird species, especially water birds, which generally coexist peacefully together. Mallards share the shores of Lake 1 with other resident water birds, including the yellow-legged gull (Larus michahellis), Eurasian coot (Fulica atra), Eurasian moorhen (Gallinula chloropus) and Egyptian goose (Alopochen aegyptiacus), while other species usually winter in the park such as the lesser black-backed gull (Larus fuscus), black-headed gull (Chroicocephalus ridibundus), common tern (Sterna hirundo), little tern (Sternula albifrons) and great cormorant (Phalacrocorax carbo). The local population of mallards was introduced in 1995, having been ceded by a protected area located near Porto. The birds were not managed, being free to leave and return to the Park. This population may also include individuals coming from other areas, including migratory ducks wintering in the lake. Mallards in the park are occasionally subject to predation by cats (Felis catus) and gulls which feed on their eggs, as well as occasionally stray dogs (Canis lupus familiaris) targeting adult individuals (data provided by Porto City Hall – Urban Parks Municipal Division).
Behavioural observations
Ad libitum observations were made before starting the systematic data collection. Based on these observations and on published literature (e.g., Cramp and Simmons 1977; Johnsgard 1960; Lorenz 1971; 1958; McKinney 1965; Ramsay 1956; Seymour and Titman 1979; Weidmann 1956), 45 different behavioural patterns were observed, as shown in the complete ethogram (Online Resource 1). These behaviours were then classified according to the following categories: Locomotion, Resting, Feeding, Comfort, Courtship Displays, Aggressive Behaviours, Defensive Behaviours and Mating (Table 1).
Mallards were observed from the 2nd of December 2015 to the 29th of April 2016, corresponding to the species’ courtship and early nesting period in Portugal (Cramp and Simmons 1977; Rodrigues 2001). During this period, mallard behaviours were examined to investigate potential variations in frequencies over time. One observation session was conducted each day, and the observation time was randomly selected between 8:00 and 14:00 h. A total of 9 h 13 min and 22 s of field observations were carried out across 34 days. The behaviour of male and female mallards was video recorded in 10 min sessions (HD Legria HF R68 camcorder equipped with a wide-angle lens, allowing to observe individuals at a greater distance), and the resulting videos were later analysed using The Observer XT 10.0 (Noldus Information Technology https://www.noldus.com/observer-xt), a specialized software for behavioural research. Behaviour frequency was determined through scan sampling (Bateson and Martin 2021) for 10-min time periods with 10 s intervals.
The distribution of the scan samples between sexes varied throughout the observation period. Weather conditions were relatively constant between all five months of observation. Due to the limited number of females in April, they were removed from interpretation (see Table 2).
As it was not possible to identify each duck individually, pseudoreplication may have occurred with the same bird being recorded multiple times on the same session; however, several measures were taken to minimize its potential impact: observation days were chosen at random between each month, introducing temporal separation between sessions; different zones within the study area were selected to record different ducks within the population and minimize the likelihood of observing the same individuals, and each group was treated as an independent observation; and scan sampling also allows for each observation to be considered an independent event. A single researcher recorded and analysed all behavioural data.
Since this particular population of mallard resides in an urban park that many people visit every day, these individuals are well accustomed to human presence and display a high level of tolerance towards human beings. This familiarity allowed them to be observed at a shorter distance than usual (50 m at most) without causing any apparent changes to their behaviour.
Our methodology has been submitted to the Ethics Committee of Instituto de Ciências Biomédicas Abel Salazar, University of Porto, for Animal Behaviour studies of their students.
Statistical analyses
Before analyses, the absolute frequency values were converted into a percentage of relative frequency values, considering the total number of observations of the individual for each session.
To distinguish male and female mallards based on their behaviour, a Student's t-test was conducted to compare the behaviour means of the two groups (Males vs. Females). To determine the most predominant food source in the mallard population, a Student's t-test was applied to compare the frequencies of eating plant matter with those of filter feeding (Grass vs. Filter).
In order to understand which physical environment was most often occupied by the population between the lake itself or its shores, a one-way ANOVA (used in order to compare the means of three or more groups) was employed to compare differences in behavioural activity among the different physical environments in which ducks were observed (Land, Water, or Air). This test (a one-way ANOVA) was also conducted to investigate the social context in which most mallards were observed (Couple, when the subject was with an individual of the opposite gender that was identified as its mate; Group, when it was together with several others of its kind; or Alone, when it was isolated from any other individuals).
To explore temporal trends in behaviours, we analysed how each behaviour of males and females varied throughout different months. To achieve this, a two-way ANOVA was conducted with Months (December, January, February, March, April), and Sex (Male, Female) as independent factors. Interactions between these two factors were tested, and if they were not significant, the models were fitted with main factors only. Multiple comparisons were Sídák adjusted. The same analysis was employed to investigate temporal variations in feeding habits, physical environments and social contexts. A Mixed-effects model was also employed in cases where missing values occurred due to random reasons. In this case, the response variables (behaviours and behaviour categories, Table 1) were tested as functions of the fixed effects sex, month, and the interaction between these two variables.
Normality and homoscedasticity were verified with Shapiro–Wilk or Kolmogorov–Smirnov test and Levene’s test respectively. The alpha level for statistical significance was set at 0.05. When the data did not meet the assumptions, nonparametric tests were used instead (such as the Mann–Whitney U Test), and when such was not possible, the alpha level was lowered to 0.01. All statistical tests were performed using GraphPad Prism version 9.0.0 for Mac (GraphPad Software, San Diego, California USA, www.graphpad.com).
Results
Activity budgets
Individuals spent the majority of time moving around their environment, with Locomotion behaviours scoring a staggering 40.51%, followed by Resting (26.21%), Feeding (13.97%) and Comfort (12.19%) (Fig. 2a). Aggressive behaviours scored 2.98% and Defensive behaviours 1.83%. Courtship displays were only observed for 2.23% of the time, and mating was the most infrequent behaviour category (0.07%) (Fig. 2b).
Proportion of a Locomotion, Rest, Feeding, and Comfort behaviour categories, and b Courtship, Aggressive, Defensive and Mating behaviour categories for the studied population of mallard (Anas platyrhynchos L.) residing in Porto City Park during the entirety of the observation period (December – April)
Throughout the sampling period, males and females were observed performing the same behavioural patterns, save certain sex-specific patterns, albeit with different frequencies.
Males were observed to perform courtship displays more often than females (males: 2.75%, females: 0.53%, U = 7676, p = 0.002) (Fig. 3a). The large difference between male and female courtship displays is mostly owed to Grunt-Whistle/ Water-Flick, Down-Up, Introductory Body-Shake and Display Comfort Behaviours – behaviours belonging to the Comfort category (e.g., Tail-Wag, Bathing) when performed in a display context, such as during a display bout – which all show significant differences between males and females (Grunt-Whistle: males = 0.31, females = 0.00, U = 8249, p < 0.05; Down-Up: males = 0.53, females 0.00, U = 8067, p < 0.01; Introductory Body-Shake: males = 0.44, females = 0.00, U = 8103, p < 0.01; Display Comfort Behaviours: males = 0.96, females = 0.03, U = 7514, p < 0.001) (Fig. 3b). From the three main male display patterns – Head-Up-Tail-Up, Down-Up and Grunt-Whistle – only Head-Up-Tail-Up did not score significant frequencies compared to the females’ null values (males = 0.10, females = 0.00, U = 9724, p = 0.126). While Nod-Swimming was also only observed in males, it was not frequent enough to show a significant difference to females (males = 0.12, females = 0.00, U = 8687, p = 0.124). Female displays were not as frequent, although they were occasionally observed Inciting, having done so significantly more than males (males = 0.00, females = 0.10, U = 8556, p < 0.01), and Head-Pumping, with no significant difference to males (males = 0.27, females = 0.43, U = 8917, p = 0.600).
Analysis of Courtship, Agonistic and Mating behaviours in this population of mallards (Anas platyrhynchos L.). a Comparison of the percentages of the Courtship Displays behaviour category, and b individual behaviours within the Courtship Displays category (± SE) which showed significant differences for female and male mallards for the entirety of the observation period. Display Comfort Behaviours include performances of behaviours that are normally associated to comfort when these were unmistakably performed in a display context. c Monthly variation in the percent of occurrences (mean ± SE) that male and female mallards allocated to Courtship Displays, and d the Mating behaviour category throughout the observation period. e Comparison of the percentages of Aggressive, and f Defensive behaviour categories (± SE) for female and male mallards for the entirety of the observation period. g Monthly variation in the percent of occurrences (mean ± SE) that male and female mallards allocated to Aggressive, h Defensive, and i the entirety of Agonistic behaviours throughout the observation period. Bars represent standard error (SE). * = p < 0.05; ** = p < 0.01; **** = p < 0.0001
The analysis of courtship displays throughout the observation period showed a main effect for Month and Sex (F(3.104, 173.0) = 3.98, p < 0.01 and F(1, 94) = 15.41, p < 0.001, respectively). Males performed more courtship displays, and the occurrence of display behaviours decreased with each passing month (Fig. 3c). December was the month during which the Courtship Display category scored the highest percentages for both males and females, with similar percentages of occurrences (males = 3.96%, females = 1.69%, t = 1.65, DF = 46.56, p = 0.432). Although there were no observations of courtship displays performed by females in January, male displays still scored high frequencies, resulting in a significant difference between male and female courtship frequencies (males = 3.64%, females = 0.00%, t = 4.29, DF = 69.00, p < 0.001). Afterwards, male displays experienced a decrease in frequency, and while they still performed displays at higher frequencies than females in February (males = 2.79%, females = 0.26%, t = 3.30, DF = 52.79, p < 0.01) the same was no longer true in March (males = 2.02%, females = 0.30%, t = 2.79, DF = 54.10, p = 0.036). No displays were observed in April by either males or females.
Mallards were sporadically observed copulating, with observations having occurred in December, February and March (Fig. 3d), however there are no significant influences of Month or Sex in mating behaviour (F(1.648, 91.86) = 1.28, p = 0.278 and F(1, 94) = 1.12, p = 0.293, respectively).
Males were also observed to perform aggressive (males = 2.38%, females = 0.00%, U = 6000, p < 0.0001) (Fig. 3e) and defensive behaviours (males = 3.12%, females = 2.54%, U = 7731, p = 0.012) (Fig. 3f) more often than females. Statistical analysis of defensive behaviours does not show a main effect for Month (F(2.455, 134.4) = 3.42, p = 0.029) nor for Sex (F(1, 94) = 0.02, p = 0.889). With the exception of February, males were consistently observed performing defensive behaviours more often than females (Fig. 3g); however, this difference was only found to be significant during the month of January (males = 3.11%, females = 0.52%, t = 3.60, DF = 87.24, p = 0.003). In February there was a peak sudden increase, albeit not significant (January = 2.41%, February = 5.48%, t = 1.77, DF = 61.00, p = 0.570), of defensive behaviours for both sexes, though more evident in females, and in March (1.65%) and April (2.03%) the observed values were similar to those in December (3.30%) and January (December vs March: t = 1.16, DF = 59.00, p = 0.945; December vs April: t = 0.61, DF = 37.00, p = 0.999; January vs March: t = 1.03, DF = 62.00, p = 0.974; January vs April: t = 0.36, DF = 37.00, p > 0.999).
Aggressive behaviour showed a main effect for Sex (F(1, 94) = 6.47, p < 0.01) but not for Month (F(1.734, 96.65) = 2.32, p = 0.111). Aggression behaviours increased in intensity starting in December (0.69%; January = 1.25%), reached a peak in February (3.65%), and afterwards decreased (March: 2.74%) until they reached a minimum in April (0.74%) (Fig. 3h). Males were statistically more aggressive than females during all months except April, while females were not observed performing aggressive patterns at any point of the observation period (December: t = 3.36, DF = 44.00, p < 0.01; January: t = 2.87, DF = 69.00, p < 0.01; February: t = 2.22, DF = 48.00, p < 0.01; March: t = 3.71, DF = 47.00, p < 0.001; April: t = 1.94, DF = 35.00, p = 0.267).
The joint analysis of aggressive and defensive categories, known together as agonistic behaviours, showed that the peak of aggressive behaviour in males, in February, corresponded to the peak of defensive behaviour in females (Fig. 3i).
Locomotion and Resting behaviour categories showed significant effects of Month (F(3.114, 246.8) = 20.85, p < 0.0001 for Locomotion; F(3345,,265.1) = 10.14, p < 0.0001 for Resting), but not for Sex (F(1, 317) = 0.40, p = 0.53 Locomotion; F(1, 313) = 3.75, p = 0.054 Resting). Comfort behaviours did not show significant effects for Month (F(3.157, 250.2) = 4.60, p = 0.013) or Sex (F(1,313) = 3.04, p = 0.082). For all individuals, the Locomotion category was at its minimum in April (17.24%), while scoring its highest value in March (66.23%); this peak in activity was found to be statistically significant compared to February (45.23%, t = 3.52, DF = 60.00, p < 0.01) as well as April (t = 10.55, DF = 37.00, p < 0.0001) (Fig. 7a). For the Comfort category, the only significant difference found was between the frequencies for December (17.67%) and January (7.68%, t = 3.79, DF = 63.00, p < 0.01) (Fig. 7b). For Resting behaviours, both males and females showed an increase for the month of January (38.50%), albeit this was not found to be significant (December: 20.23%, t = 3.25, DF = 63.00, p = 0.343). Later, a significant difference was found between the months of March (10.78%) and April (35.38%, t = 4.16, DF = 37.00, p < 0.01), as males were observed resting more often than females, who in turn scored much lower values than in previous months (Males: 37.30%; Females: 0.82%; t = 5.60, DF = 35.81, p < 0.0001) (Fig. 7c) (Fig. 4).
Feeding habits
The population’s feeding habits were analysed, and individuals were observed feeding on grass more often than filtered particles (55.31% vs 27.32%, t = 5.54, R2 = 0.09, p < 0.0001) (Fig. 5a); this was true for males (54.08% vs 26.52%, t = 4.74, R2 = 0.08, p < 0.0001), as well as for females (59.26% vs 29.91%, t = 2.85, R2 = 0.10, p < 0.01).
Analysis of the population’s feeding habits. a Comparison of percentages between grass and filter feeding for the entirety of the observation period. b Monthly variation in the percent of occurrences (mean ± SE) that male and female mallards allocated to total Feeding behaviours, c grass, and d filter feeding for both males and females throughout the observation period. The percent of feeding occurrences (b) considers the total number of activities performed by either sex for each month as 100%. The percent of grass feeding (c) and filter feeding (d) occurrences considers the total occurrences of feeding behaviours performed by either sex for each month as 100%. Bars represent standard error (SE). **** = p < 0.0001
The relative percentages of feeding behaviours change with each passing Month (F(2.954, 234.1) = 7.96, p < 0.001) (Fig. 5b). Females generally fed more often than males (F(1, 317) = 9.54, p < 0.01), scoring a significantly higher value in December (December: females = 28.60%, males = 6.86%, t = 2.68, DF = 20.07, p < 0.05; January: females = 20.52%, males = 17.47%, t = 0.43, DF = 37.59, p = 0.673; February: females = 21.24%, males = 7.62%, t = 1.53, DF = 13.33, p = 0.150; March: females = 4.10%, males = 3.17%, t = 0.30, DF = 19.06, p = 0.777; April: females = 52.46%, males = 25.95% ± , t = 0.62, DF = 1.04, p = 0.645). The number of occurrences of the feeding of males decreased significantly from January to March (t = 3.60, DF = 47.00, p < 0.001) and then increased in April (t = 4.04, DF = 35.00, p < 0.01).
Statistical analysis of grass feeding showed a main effect for Month (F(3.20, 78.40) = 15.98, p < 0.001), displaying an inverse pattern to that of filter feeding (Fig. 5c). Grass feeding was the most common type of feeding throughout the year except for March, when the ducks opted for filter feeding instead. The frequency at which individuals resorted to filter feeding also showed a significant effect for Month (F(3.007, 76.69) = 23.23, p < 0.0001). However, no Sex differences were found for filter feeding (F(1, 52) = 0.32, p = 0.574) (Fig. 5d). Individuals were observed filter feeding more frequently in December (35.19%) compared to January (3.85%, t = 4.06, DF = 33.00, p < 0.001), as well as in March (86.92%), displaying a distinctive peak compared to February (15.99%, t = 10.45, DF = 19.00, p < 0.0001) and April (30.57%, t = 4.72, DF = 17.00, p < 0.001).
Activity in different physical environments
Behavioural analysis showed that activities were different throughout physical environments (F(2,966) = 169.00, p < 0,0001). Mallards performed behaviours on land and in water with similar percentages (t = 1.02, p = 0.667). Ducks were only sporadically observed flying, leading to a small frequency for behaviours observed in the air (0.94%), significantly lower than that of water (51.09%, t = 16.41, p < 0.0001) and land (47.97%, t = 15.39, p < 0.0001) (Fig. 6a).
Analysis of the population’s activity in different physical environments. a Comparison of the percentages of behaviours observed in water, on land and through the air for the entirety of the observation period. R2 = 0.10. b Monthly variation in the percent of occurrences (mean ± SE) that male and female mallards allocated to behaviours performed in water, c land, and d air throughout the observation period. Bars represent standard error (SE). **** = p < 0.0001
A main effect for Month was found for both the percentage of mallard behaviours in water and for those on land (F(3.312, 184.6) = 9.19, p < 0.0001 and F(3.274, 182.5) = 11.28, p < 0.0001, respectively). Individuals were observed in the lake more frequently in March (77.01%), but the frequency of behaviours in water later decreased, reaching a minimum in April (25.01%) (Fig. 6b). As occurrences of activities on land are dependent on occurrences in water, we observed that the percentage of occurrence of behaviours on land were at their lowest in March (19.12%) and reached a maximum in April (74.74%), conversely to what had been observed for the occurrence of behaviours in water (Fig. 6c).
Individuals were observed flying through the air in December, and again in February (only males), March and April. However, mallards were only observed in the air sporadically, and as such there is no observable pattern of progression throughout the observation period (Month: F(1.111, 61.95) = 2.67, p = 0.104), nor any differences between sexes (Sex: F(1, 94) = 0.90, p = 0.344) (Fig. 6d).
Activity in different social contexts
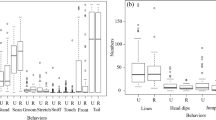
Behavioural activity is significantly affected by social context (F(2, 966) = 791.20, p < 0.0001). The vast majority of individuals was most often observed forming groups, leading to the Group social context scoring the highest among the three categories (77.75%) (Fig. 7a). Behaviours performed in a couple with their respective mates scored the lowest frequency out of the three (6.57%), lower than that of activities observed alone (14.89%). All frequencies were found to be statistically different between one another (Alone vs. Couple: t = 6.01, p < 0.0001; Alone vs. Group: t = 45.43, p < 0.0001; Couple vs. Group: t = 51.45, p < 0.0001).
Analysis of the population’s activity in different social contexts. a Comparison of the percentages of behaviours performed in a group, in a couple and alone for the entirety of the observation period. R2 = 0.62. Monthly variation in the percent of occurrences (mean ± SE) that male and female mallards allocated to behaviours observed b alone, c in a couple and d in a group throughout the observation period. Bars represent standard error (SE). **** = p < 0.0001
The activity of ducks observed alone was not affected significantly by time of year (F(2.887, 161.0) = 2.75, p = 0.047) or sex (F(1, 94) = 0.87, p < 0.352). Despite not having a significant interaction between Sex and Month, we observed that, in March and April, males scored increasingly higher frequencies for the Alone social context (March: 22.14% April: 24.35%) (Fig. 7b).
There was no temporal pattern observed for behaviours that were performed by individuals in a couple (F(2.410, 191.0) = 2.83, p = 0.052). However, the overall percentage of the Couple category was found to be statistically higher for females than for males (F(1, 317) = 14.84, p < 0.0001), despite neither of the values being significantly different from one another for each individual month (December: 3.54% vs 2.18%, t = 0.82, DF = 28.17, p = 0.413; January: 18.38% vs 6.69%, t = 1.89, DF = 33.68, p = 0.067; February: 10.39% vs 2.96%, t = 1.11, DF = 14.11, p = 0.285; March: 22.15% vs 6.84%, t = 1.66, DF = 17.40, p = 0.115; April: 15.58% vs 1.11%, t = 1.35, DF = 1.01, p = 0.403) (Fig. 7c).
Statistical analysis reveals no Sex differences in the percentage of occurrences that mallards allocated to behaviours in group (F(1, 313) = 0.206, p = 0.650). The frequency of group behaviours was also found to not differ significantly throughout the observation period, having consistently scored high values during all five months of observation (F(3.276, 256.3) = 3.42, p = 0.015) (Fig. 7d).
Discussion
This research has identified marked differences in activity patterns between the mallards’ courtship and nesting periods in Portugal. Our results reflect the behavioural differences between sexes during these periods, with males being responsible for most of the courtship and aggressive frequencies while females foraged in preparation to egg-laying. Overall, the time budget in this population was similar to what has been described for other urban populations of mallard as well as other dabbling ducks. Several adaptations to an anthropized environment were found, including lower defensive behaviours as well as higher feeding rates. Our results are also in line with other populations in mainland Portugal.
The number of individuals observed in the communal area for this population of mallards varied each day, progressively decreasing every month. We also consistently observed more males than females throughout the observation period. Both these phenomena have been previously reported for wild populations of mallard; Raitasuo (1964, in Weidmann and Darley 1971a) claims that mallard populations are normally composed of more males than females, while Ryser and Dewey (1985) observed that paired couples gradually disperse from the communal areas, retreating to their nesting areas. Pairing was successful for the majority of individuals in the Porto City Park population, with most females having left the communal area and nested in nearby locations such as small retreated islets within the lake (ad libitum observations), and by the end of the observation period only a fraction of the population remained in the lake shore, mostly unpaired males. This difference can also be partially attributed to non-resident individuals abandoning the site after their wintering period, though their proportion compared to resident individuals is unknown.
Activity budgets
Courtship displays decreased with time, most noticeably for males. As more pairs formed and left the communal area, fewer females became available, thus leading to a decline in display intensity as the courtship period progressed. These results reflect what was observed by Lee (1985), who noted that courtship reached a peak in intensity in mid-winter, during pair formation. Males scored impressively high courtship frequencies compared to females, owing to their highly competitive and ritualized displays, which they perform synchronously in large aggregations and repeat several times in sequence in coordinated “bursts” (Williams 1983). As such, it is expected that male displays would score higher frequencies than those of females.
Aggressive behaviour was only observed in males for the entirety of the observation period. Aggression in male mallards has been widely documented (Barash 1977; Barrett 1973; Goodwin 1956; Hailman et al. 1978; McKinney 1965; McKinney et al. 1978; Seymour and Titman 1979; Weidmann 1956). Female mallards do not usually display aggression towards other individuals, although this is not entirely unheard of; for instance, Darbouze et al. (2016) noted that females would bite males if they got too close, though this observation may be attributed to overcrowding, as the authors observed an excess of individuals in their study locations. The low frequencies of aggressive behaviours in the present study, as well as the absence of female aggression, could be signs that this population is not subject to overcrowding.
The low frequencies of defensive behaviours observed may be a result of habituation to humans and lower predation pressure, as is known from captive and urbanized waterbirds, which are described as having reduced alert and fleeing behaviour compared to wild populations (Donaldson et al. 2007; Luniak 2004; Rose et al. 2022; Traut and Hostetler 2003). Even accounting for other defensive patterns performed as a response to intraspecific aggression, their low overall value shows that the population in Porto City Park is habituated to human presence.
Aggressive and defensive behaviours were not affected by the months passing, which is in accordance to Lee (1985), who observed no temporal variation in agonistic activities throughout the observation period. Male aggression initially scored relatively low frequencies, reflecting how paired males are not especially protective of their mates during the first stages of pair formation (Seymour and Titman 1979); later, male aggression became more frequent, and in February both aggressive and defensive frequencies peaked. Our results suggest that the decreasing number of available females drives males into becoming more aggressive, competing more intensely for them and chasing them more often, leading to a corresponding increase in defensive responses. February was also the month during which reproductive behaviour (Mating) scored the highest frequencies, further hinting at February corresponding to the peak of the courtship period. The decrease in aggressive and defensive behaviours in latter months reflects how couples left the communal area for nesting, leading to fewer individuals being present and thus fewer situations that would trigger aggressive behaviours and subsequent defensive responses.
The three most frequent activities throughout the observation period were Locomotion, Rest and Feeding, with Comfort behaviours taking up a slightly lower proportion to Feeding. These findings are mostly consistent with what has been observed in both captive and wild ducks (Munday and Rose 2022; Rose et al. 2022; Tamisier 1976); despite our similarities with these time budgets, Tamisier (1976) found feeding to be “insignificant”. This disparity may be due to differences in food availability and predation pressure from urbanized environments to the wild; moreover, Tamisier’s (1976) observations reflect the time budget of a wintering (migratory) population, while our study mainly observes the behaviour of resident ducks.
Locomotion and Rest showed temporal differences throughout the observation period. The changes in Locomotion can be explained by several factors, including courtship intensity (Weidmann and Darley 1971b), feeding habits (Houhamdi and Samraoui 2008), the increase in aggressive and defensive behaviours leading to ducks avoiding each other, and other activities. The distinct drop in Locomotion frequencies in April reflects the end of the courtship period, and how much more active the species is throughout its duration.
The progression of Resting behaviours reflects the progression of the species' annual cycle. Anatidae are known to be significantly more active during the breeding season compared to the wintering period (Munday and Rose 2022), which is reflected in their January peak and progressive decrease until March. The high values observed in January may also be related to weather conditions, since this was the month with the most rain and highest humidity value (data from timeanddate.com). These results are according to the literature, as ducks are known to seek shelter and rest more during periods of heavy rain (Munday and Rose 2022). The dramatic increase in resting from March to April reflects the end of the courtship period, with unpaired males becoming less active due to the lack of available females.
Comfort activities remained constant throughout the observation period, which is to be expected since comfort behaviours are daily activities that are required for the maintenance of the individual’s hygiene and wellbeing (McKinney 1965).
The population’s time budget is similar to what has been observed in wild populations (McKinney 1967, in Bruggers and Jackson 1977), and roughly follows the same phenology as other Portuguese populations of mallards (Rodrigues 2001). Nevertheless, their adaptation to this anthropized environment can be noticed in some aspects of their behaviour, including a higher frequency of feeding and less frequent defensive behaviours.
Feeding habits
Generally, females spent more time feeding than males, which may be due to the fact that males were observed performing the Alert behaviour more often than females; males in small groups would stay alert for long periods of time while females fed. This was also observed by Bruggers and Jackson (1977) as well as Kaminski and Prince (1981), and was interpreted as the males watching over the females, allowing them more time to feed. Additionally, Asplund (1981) found that pre-laying female Anseriformes feed more often than males, as egg production requires a greater nutrient intake. While it is impossible to calculate the proportion of females in the pre-laying stage based on the difference of values observed for the two sexes, we can attribute this difference to their presence.
The seasonal fluctuations of the mallard’s feeding habits have been attributed to the quality of food sources and their availability on a temporal and spatial level, as well as different physiological needs throughout their annual cycle (Paulus 1988; Tidwell et al. 2013). In our study, mallards were found to feed on grass more often than filtered particles; this pattern was observed during all months except March, where filter feeding was the dominant food source. For most dabbling ducks, winter is characterized by ample consumption of high-energy plant matter, with invertebrate foods becoming more prevalent during spring when females require greater protein intake for egg formation (Gammonley 1995; Hohman et al. 1996; Krapu 1974; Reinecke and Owen 1980). The decrease in filter feeding observed in April can be mainly explained by the dispersal of paired females from the communal area. As for why a similar effect is also observed for males, one possibility is that males would behave similarly to females in order to stay close to them, feeding on the same food sources as them. These and other factors – weather conditions leading to a more humid environment and the formation of more puddles, abundance of invertebrates in the water, similar nutritional needs for males and non-gestating females – may explain why males and females were found to feed on both grass and filtered particles in similar proportions.
Activity in different physical environments
The frequency of activities observed on land and in water changed with the progression of the observation period. One of the main factors to take into account when understanding habitat use in Anatidae are weather conditions (Paulus 1988). In the literature (Finney et al. 1999; Munday and Rose 2022; Shao and Chen 2017; YiJin et al. 2019), swimming rates increase with lower temperatures. A similar pattern was observed in our study for March, which was not only the coldest month of observation but also the one in which behaviours observed in water reached a peak (see Table 2). Interestingly, environment occupation in our population seem to mirror the previously observed changes for the consumption of different food sources; the evolution of filter feeding and grazing frequencies are strikingly similar to those of behaviours observed in water and on land, respectively, which are also the physical environments where ducks are more likely to procure these respective food sources. Feeding habits play a significant role in habitat use, with individuals spending more time on land or water as they forage for specific foods according to their availability and physiological needs. It is also important to note that courtship as a whole is heavily linked to water; mallards will gather in the water to perform or watch courtship displays in large numbers, and the males’ three main display patterns are always performed in water. Despite displays and courtship behaviours only accounting for about 2% of the total observed behaviours in this study, much time during courtship was spent between display bouts, and individuals would still linger in the water after the displays had been performed for the moment, dispersing and resuming their daily activities. The fact that so few individuals were observed in water during the month of April compared to other months may be owed to the fact that no courtship displays were observed for this month. There are also other behavioural patterns and activities that are exclusively performed either in water or on land, such as Bathing or Sleeping, all of which play a role in how these frequencies changed with time. Ultimately, we must consider that the progression of habitat use in this population is determined by a combination of all these different factors, and possibly more.
Although individuals were also observed flying, these behaviours were quite infrequent, and ducks were never observed flying for long periods of time; individuals would fly away/past the observation area or otherwise landed shortly afterwards. It is common to observe males chasing females through the air intending to copulate with them during the first weeks of the incubation period (Cramp and Simmons 1977). McKinney (1967) found that the frequency of these flights increases immediately before egg-laying. In fact, this tendency was detected for the months of February and March, reaching a peak in the latter month, before most females had retreated to incubate their eggs. Still, the total frequency of airborne behaviours may be an underestimate for the population, as is observed in most studies in Anatidae (Paulus 1988).
Activity in different social contexts
Owing to the species’ gregarious nature and to the highly social character of courtship, activities performed in groups dominated the observation period, with no differences found between male and female frequencies. Mallards flock together with their conspecifics for protection, and a greater number of individuals can more easily spot intruders and predators. Throughout the observation period, activities performed in groups remained relatively constant, since even as more couples were formed, there were always individuals – mostly unpaired males – that spent most of their time gathered in small groups. Similar to what had been observed by Seymour and Titman (1979), these congregations of unpaired males stayed in the communal area by the shores of the lake after couples had left for their nesting areas.
The percentage of occurrences of behaviours performed in a couple was the lowest among the three social contexts. In this population, the greater number of males compared to females means that several males were left with no females to form couples with and remained unpaired, leading to a difference in behaviours observed in a couple for both sexes. Although the number of pairs increased each passing month, the frequencies of behaviours observed in a couple remained constant throughout the observation period. Pair formation does not necessarily entail a correspondent linear rise in activities performed in couples from one month of observation to the other, since coupled individuals do not spend the totality of their time with their mate. Since both scan samples of females recorded in April were observed with an isolated male away from other individuals, we can conclude that no females were left unpaired in the population.
As for behaviours that were performed alone, their frequencies were lower than those observed in a group. Males spent more and more time by themselves as the observation period progressed, which may be connected to the decline of courtship intensity that was previously described. In contrast, females were observed spending less time alone from February onward, which could be indicative of not only pair formation but also the lower number of females observed for the two last months of observation.
Conclusions
The results in this paper may help understand the behavioural and ecological adaptations of mallard populations from Southwestern Europe. Future studies ought to further investigate the daily activities and population dynamics – including breeding success and survival rates – of similar populations in the southwest of Europe, as well as of other individuals and populations inhabiting urban parks and other settings where familiarity with humans and external disturbances are more common.
Data availability
The data supporting the findings of this study are available upon request from the corresponding author, Ana Magalhães.
References
Abraham RL (1974) Vocalizations of the mallard (Anas platyrhynchos). The Condor 76(4):401–420. https://doi.org/10.2307/1365814
Afton AD (1979) Time budget of breeding Northern Shovelers. Wilson Bull 91(1):42–49
Asplund C (1981) Time budgets of breeding mallard in northern Sweden. Wildfowl 32(32):55–64. https://wildfowl.wwt.org.uk/index.php/wildfowl/article/view/628/628. Accessed 25 Feb 2022
Avilova KV, Scobeyeva VA, Artyushin IV, Golubeva TB, Fokin SY (2023) Behavioral and genetic isolation of the urban mallard population (Anas platyrhynchos) in Central Russia: Myth or Reality? Biol Bull Rev 13(3):205–215
Barash DP (1977) Sociobiology of rape in mallards (Anas platyrhynchos): responses of the mated male. Science 197(4305):788–789. https://doi.org/10.1126/science.197.4305.788
Barrett J (1973) Breeding behavior of captive mallards (No. COO-1332-92). Dissertation, University of Minnesota Press, Minneapolis, United States. https://doi.org/10.2172/4455539
Bateson M, Martin P (2021) Measuring behaviour: an introductory guide, 4th edn. Cambridge University Press, Cambridge, UK
Bennett MJ (1987) Comparison of the diet, feeding behavior, and habitat use of mallards (Anas platyrhynchos) and black ducks (Anas rubripes). Biology Honors Papers 18. https://data.jncc.gov.uk/data/08cfb4da-4c5a-4bef-b45d-8f2f87dc8070/waterbirds-around-the-world.pdf
Bruggers RL, Jackson WB (1977) Time budgets of Mandarin Ducks under semi-natural conditions. Wildfowl 28(28):7
Carvalho D, Horta P, Raposeira H, Santos M, Luís A, Cabral JA (2013) How do hydrological and climatic conditions influence the diversity and behavioural trends of water birds in small Mediterranean reservoirs? A community-level modelling approach. Ecol Model 257:80–87
Cheng KM, Burns JT, McKinney F (1982) Forced copulation in captive mallards (Anas platyrhynchos): II. Temporal Factors Animal Behaviour 30(3):695–699. https://doi.org/10.1016/S0003-3472(82)80140-1
Costa LT, Guedes RS (1994) Contagens de anatídeos invernantes em Portugal-1992–93. Estudos de Biologia e Conservação da Natureza, 14. Instituto da Conservação da Natureza, Lisbon, Portugal
Cramp S, Simmons KEL (1977) The birds of the western Palearctic, vol 1. Oxford University Press, Oxford, United Kingdom, Ostrich to Ducks
Cruz T, Lima J, Luis A (2021) Estudo de reprodução de Pato-real (Anas platyrhynchos). Revista Captar: Ciência e Ambiente Para Todos 10:5–5
Darbouze G, Volpe C, Leedom LJ (2016) Fascinating social displays and social structure of mallard ducks (Anas platyrhynchos). Department of Psychology, University of Bridgeport, Bridgeport, CT. https://core.ac.uk/reader/52956760. Accessed 22 Feb 2022
Delacour J (1956) The waterfowl of the world, vol. 2. The dabbling ducks. Country Life
Donaldson MR, Henein KM, Runtz MW (2007) Assessing the effect of developed habitat on waterbird behaviour in an urban riparian system in Ottawa, Canada. Urban Ecosyst 10:139–151
Downs J, Loraamm R, Anderson JH Jr, Perry J, Bullock J (2017) Habitat use and behaviours of introduced Muscovy ducks (Cairina moschata) in urban and suburban environments. Suburban Sustainability 5(1):1. https://doi.org/10.5038/2164-0866.5.1.1028
Dwyer TJ (1975) Time budget of breeding Gadwalls. The Wilson Bulletin 87(3):335–343
Engel J, Keller M, Leszkowicz J, Zawadzki J (1988) Synurbization of the mallard Anas platyrhynchos in Warsaw. Acta Ornithologica 24(1):9–28
Fernández-Juricic E, Telleria JL (2000) Effects of human disturbance on spatial and temporal feeding patterns of Blackbird Turdus merula in urban parks in Madrid, Spain. Bird Study 47(1):13–21
Finney SK, Wanless S, Harris MP (1999) The effect of weather conditions on the feeding behaviour of a diving bird, the Common Guillemot Uria aalge. J Avian Biol 30(1):23–30. https://doi.org/10.2307/3677239
Gammonley JH (1995) Spring feeding ecology of cinnamon teal in Arizona. Wilson Bull 107(1):64–72
Goodwin D (1956) Displacement coition in the mallard. Br Birds 49:238–240
Hailman JP, Dzelzkalns JJI (1974) Mallard Tail-Wagging: punctuation for animal communication? Am Nat 108(960):236–238. https://doi.org/10.1086/282904
Hailman JP, McKinney F, Barrett J, Derrickson SR, Barash DP (1978) Rape among mallards. Science 201(4352):281–282. https://doi.org/10.1126/science.201.4352.280
Hohman WL, Stark TM, Moore JL (1996) Food availability and feeding preferences of breeding fulvous whistling-ducks in Louisiana ricefields. Wilson Bull 108(1):137–150
Houhamdi M, Samraoui B (2008) Diurnal and nocturnal behaviour of Ferruginous duck Aythya nyroca at Lac des Oiseaux, northern Algeria. Ardeola 55(1):59–69. https://www.ardeola.org/uploads/articles/docs/1365.pdf. Accessed 10 Mar 2022
Johnsgard PA (1960) A quantitative study of sexual behavior of mallards and black Ducks. Wilson Bull 72(2):133–155
Kaminski RM, Prince HH (1981) Dabbling duck activity and foraging responses to aquatic macroinvertebrates. Auk 98(1):115–126. https://doi.org/10.2307/3807868
Krapu GL (1974) Feeding ecology of pintail hens during reproduction. Auk 91(2):278–290. https://sora.unm.edu/sites/default/files/journals/auk/v091n02/p0278-p0290.pdf. Accessed 4 Apr 2016
Lebret T (1961) The pair formation in the annual cycle of the mallard, Anas platyrhynchos L. Ardea 49(3–4):97–158
Lee SD (1985) A time budget study of mallards on the Texas High Plains. Dissertation, Texas Tech University. https://ttu-ir.tdl.org/bitstream/handle/2346/8918/31295003974945.pdf?sequence=1&isAllowed=y. Accessed 16 Feb 2022
Liordos V (2010) Foraging guilds of waterbirds wintering in a Mediterranean coastal wetland. Zool Stud 49(3):311–323
Lorenz KZ (1958) The evolution of behavior. Sci Am. https://doi.org/10.1038/scientificamerican1258-67
Lorenz KZ (1971) Comparative studies of the motor patterns of Anatinae. Stud Anim Human Behav 2:14–114. https://doi.org/10.4159/harvard.9780674430426.c4
Lowry H, Lill A, Wong BB (2013) Behavioural responses of wildlife to urban environments. Biol Rev 88(3):537–549
Luniak M (2004) Synurbization–adaptation of animal wildlife to urban development. Proceedings 4th international urban wildlife symposium. Tucson, University of Arizona, pp 50–55
McKinney F (1965) The comfort movements of Anatidae. Behaviour 25(1):120–220. https://doi.org/10.1163/156853965X00138
McKinney F, Barrett J, Derrickson SR (1978) Rape among mallards. Science 201(4352):281–282. https://doi.org/10.1126/science.201.4352.281
McKinney F (1967) Breeding behaviour of captive Shovelers. Wildfowl 18(18):108–121. https://wildfowl.wwt.org.uk/index.php/wildfowl/article/view/318. Accessed 15 May 2016
Morelli F, Mikula P, Benedetti Y, Bussière R, Jerzak L, Tryjanowski P (2018) Escape behaviour of birds in urban parks and cemeteries across Europe: evidence of behavioural adaptation to human activity. Sci Total Environ 631:803–810
Munday C, Rose P (2022) Environmental and social influences on the behaviour of free-living mandarin ducks in richmond park. Animals 12(19):2554
Parejo M, Navedo JG, Gutiérrez JS, Abad-Gómez JM, Villegas A, Corbacho C, Sánchez-Guzmán JM, Masero JA (2015) Geographical origin of dabbling ducks wintering in Iberia: sex differences and implications for pair formation. Ibis 157(3):536–544
Paulus SL (1988) Time-activity budgets of nonbreeding Anatidae: a review. Waterfowl in winter. University of Minnesota Press, Minneapolis, United States, pp 135–152
Raitasuo K (1964) Social behaviour of the mallard, Anas platyrhynchos, in the course of the annual cycle. Papers on Game Research 24:1–72
Ramsay AO (1956) Seasonal patterns in the epigamic displays of some surface-feeding ducks. Wilson Bull 68(4):274–281
Reinecke KJ, Owen RB Jr (1980) Food use and nutrition of black ducks nesting in Maine. J Wildl Manag 44(3):549–558. https://doi.org/10.2307/3808002
Rodrigues DJC, Fabião A (1997) Loss and change of habitat and possible effects on mallard populations of Mondego and Vouga river basins. ITE Symposium. Institute of Terrestrial Ecology 30:127–130
Rodrigues DJC (2001) Ecologia e Ordenamento de Populações de Pato-real (Anas platyrhynchos L.) em zonas húmidas de Portugal. Dissertation, Instituto Superior de Agronomia, Lisbon
Rodrigues DJC, Fabião A, Figueiredo ME, Tenreiro PJ (2000) Migratory status and movements of the Portuguese Mallard (Anas platyrhynchos). Die Vogelwarte, 292–297. http://hdl.handle.net/10400.5/1361
Rodrigues DJC, Figueiredo M, Fabião A (2002) Mallard (Anas platyrhynchos) summer diet in central Portugal rice fields. Game Wildl Sci 55–62. ISSN 1622-7662
Rodrigues DJC, Figueiredo ME, Fabião A, Tenreiro PJ (2006) Waterfowl marking in Portugal: main results and future perspectives. In: Boere GC, Galbraith CA, Stroud DA (eds) Waterbirds around the world, TSO Scotland Ltd, Edinburgh, UK, pp 474–475. Proceedings of the 'Waterbirds around the World Conference', held in Edinburgh in April 2004. https://data.jncc.gov.uk/data/08cfb4da-4c5a-4bef-b45d-8f2f87dc8070/waterbirds-around-the-world.pdf
Rose PE, Roper A, Banks S, Giorgio C, Timms M, Vaughan P, Hatch S, Halpin S, Thomas J, O’Brien M (2022) Evaluation of the time-activity budgets of captive ducks (Anatidae) compared to wild counterparts. Appl Anim Behav Sci 251:105626. https://doi.org/10.1016/j.applanim.2022.105626
Rufino R (1989) Atlas das aves que nidificam em Portugal Continental. Ministério do Plano e da Administração do Territorio, Serviço Nacional de Parques, Reservas e Conservação da Natureza. ISBN: 978-972-37-1374-9
Ryser FA, Dewey J (1985) Mallard. In: Ryser FA (ed) Birds of the Great Basin: a natural history. University of Nevada Press, pp 140–147
Saez-Royuela S, Martinez S (1985) Estudio sobre la biología migratoria del orden anseriformes (aves) en España. SEO, Madrid, Spain
Seymour NR, Titman RD (1979) Behaviour of unpaired male black ducks (Anas rubripes) during the breeding season in a Nova Scotia tidal marsh. Can J Zool 57(12):2421–2428. https://doi.org/10.1139/z79-314
Shao M, Chen B (2017) Effect of sex, temperature, time and flock size on the diving behavior of the wintering Scaly-sided Merganser (Mergus squamatus). Avian Res 8(1):1–7. https://doi.org/10.1186/s40657-017-0067-4
Simmons KEL, Weidmann U (1973) Directional bias as a component of social behaviour with special reference to the mallard, Anas platyrhynchos. J Zool 170(1):49–62. https://doi.org/10.1111/j.1469-7998.1973.tb05043.x
Tamisier A (1976) Diurnal activities of green-winged teal and pintail wintering in Louisiana. Wildfowl 27(27):19–32
Tidwell PR, Webb EB, Vrtiska MP, Bishop AA (2013) Diets and food selection of female mallards and blue-winged teal during spring migration. J Fish Wildl Manag 4(1):63–74. https://doi.org/10.3996/072012-JFWM-062
Traut AH, Hostetler ME (2003) Urban lakes and waterbirds: effects of development on avian behavior. Waterbirds 26(3):290–302
Weidmann U (1956) Some experiments on the following and the flocking reaction of mallard ducklings. Br J Anim Behav 4(2):78–79. https://doi.org/10.1016/s0950-5601(56)80031-2
Weidmann U, Darley J (1971a) The role of the female in the social display of mallards. Anim Behav 19(2):287–298. https://doi.org/10.1016/S0003-3472(71)80009-X
Weidmann U, Darley J (1971b) The synchronization of signals in the “social display” of Mallards. Rev Comp Anim 5:131–135
Williams DM (1983) Mate choice in the mallard. Mate choice. Cambridge University Press, Cambridge, pp 297–309
YiJin Z, MingQin S, Peng C, Bin C (2019) Time budget and activity rhythm of the mandarin duck Aix galericulata in the Poyang Lake Watershed. Pak JZool 51:725–730. https://doi.org/10.17582/journal.pjz/2019.51.2.725.730
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study complied Portuguese legal requirements.
Competing interest
All authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santos, L.E., de Sousa, L. & Magalhães, A. Time budget of a mallard duck population residing in an urban park in northern Portugal during courtship and nesting period. acta ethol (2024). https://doi.org/10.1007/s10211-024-00436-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10211-024-00436-w