Abstract
Traffic noise is a pervasive pollutant that affects wildlife at individual and group levels through mechanisms such as disrupting communication, affecting antipredator strategy, and/or changing how they use space within a habitat. Urbanization is expanding rapidly—few places remain untouched by anthropogenic noise disturbance—so understanding the implications of noise on wildlife behavior is paramount to conservation efforts. We asked whether traffic noise could change space use and social network metrics in flocks of captive birds. Specifically, we quantified the effects of playbacks of traffic noise on individual sociality (weighted degree, number of social partners weighted by the frequency of interactions with those social partners) and flock clustering (global clustering coefficient, connectivity of neighbors). In this study, we recorded social interactions and space use of flocks of captive zebra finches (Taeniopygia guttata) before, during, and after an experimental traffic noise introduction in two treatments: high- and lower-amplitude noise. Our results demonstrated that individual sociality and flock clustering increased in response to the noise introduction in both high-amplitude and low-amplitude treatments. Additionally, birds in the high-amplitude treatment spent more time in the room with active playback during noise playback whereas birds in the lower-amplitude treatment decreased time spent in the room closest to the high-amplitude treatment. Increased social behavior in response to traffic noise could influence disease transmission, social learning, and mating dynamics. We suggest future studies explore the mechanisms driving increased social behavior in traffic noise, such as perceived predation risk, vigilance, and cross-sensory interference.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urban areas create unprecedented pressures for the wildlife that dwell in them (Sih et al. 2016). Among those pressures is noise pollution, which affects how animals communicate (Brumm and Slabbekoorn 2005), form pair bonds (Swaddle and Page 2007a), forage and protect themselves against predators (Wale et al. 2013; Jung et al. 2020; Sweet et al. 2022), and use space (McClure et al. 2013; Chen and Koprowski 2015; Jung et al. 2020; Liu et al. 2020). Given the known effects of noise on individual and group-driven behaviors (Bruintjes and Radford 2013), we suspect these effects of noise compound to change how individuals and groups interact with each other at the social network level (i.e., social behavior).
Some avian species alter their habitat use and spatial distribution along noise gradients, by choosing to settle in quieter areas (Injaian et al. 2018a; Gomes et al. 2021) or decreasing abundance as noise levels increase (McClure et al. 2013; Chen and Koprowski 2015). When given the choice between a high-amplitude noise environment and a quieter, low-amplitude noise environment in captive settings, previous work demonstrates that birds will either spend more time in the low-amplitude environment (Liu et al. 2020) or change their behavior in the high-amplitude noise environment (Kight and Swaddle 2015; Derryberry et al. 2017; Evans et al. 2018). Changes in avian behavior in response to noise included altering the frequency or amplitude of songs, or even changing the timing of dawn chorus to avoid commuter traffic (Fuller et al. 2007; Kight and Swaddle 2015; Derryberry et al. 2016, 2020; Luther et al. 2016; Zwart et al. 2016). Traffic noise is a prevalent source of noise pollution in urban areas; however, there is spatial and temporal variation in the intensity of traffic noise (e.g., varying road traffic on highways vs backroads, times of rush-hour traffic). To date, many studies of noise pollution have compared extreme ends of an amplitude gradient (i.e., noise-added compared with no-noise added; cf. (McClure et al. 2013; Ware et al. 2015). However, given the spatial and temporal heterogeneity of traffic noise, it is important to understand how variation in noise intensity influences wildlife and their behavior. Such scenarios may better represent the variation in exposure to noise that urban-dwelling species experience.
For animals that remain in high-amplitude noise conditions, noise can mask acoustic signals and disrupt communication if there is a frequency and/or temporal overlap between the noise and the signals. Tactics to compensate for masking effects in noise include adjusting vocal frequency and/or vocal performance (rate of sounds being produced given their frequency bandwidth) and/or amplitude to increase detection (Brumm 2004; McMullen et al. 2014; Kight and Swaddle 2015; Luther et al. 2016 Derryberry et al. 2020) or vocalizing at different times of day (Fuller et al. 2007; Cartwright et al. 2014). Physiologically, noise can alter baseline stress hormones and/or stress response in the individuals exposed to it, although the changes to glucocorticoid profiles appear to be species-dependent (Crino et al. 2013; Angelier et al. 2016; Davies et al. 2017; Injaian et al. 2018b; Zollinger et al. 2019). Additionally, noise creates cross-sensory interference and can affect visual and olfactory processing (Chan et al. 2010; Kunc et al. 2014; Morris-Drake et al. 2016; Halfwerk and Van Oers 2020; Zhu et al. 2022).
Disruptions to normal social behavior can have wide implications for other processes, as social behavior can modulate territory defense (Nowicki et al. 1998), mating decisions (Poesel et al. 2006; Baldassarre et al. 2016), and how information spreads within groups (Urban et al. 2011). Additionally, individuals within a population could respond to disturbance differently depending on their personality traits (e.g., explorer vs sedentary, aggression levels) (Grunst et al. 2019; Harding et al. 2019). Changes to individuals can have group-level consequences for social networks, including affecting the connectivity among individuals in the network or the group’s ability to access resources (Balasubramaniam et al. 2021; Wiśniewska et al. 2022). Changes to sensory information processing and communication could therefore influence social dynamics independently of conspecific density and space use.
Our knowledge regarding the effects of disturbance on social networks is expanding, receiving more attention in recent years. For example, mixed-species groups of large herbivores increase social affinity in response to high rainfall levels (Meise et al. 2019), and simulation-based models reveal that social connectivity of elephant herds decreases in response to targeted poaching of larger, older individuals (Wiśniewska et al. 2022). In free-living social birds, the experimental introduction of traffic noise in an otherwise quiet area resulted in decreased social interactions within and among cooperatively breeding groups (Hawkins et al. 2020).
In this study, our main goal was to investigate how noise could change characteristics of social behavior, including individual sociality and group-level social clustering in birds chronically exposed to elevated ambient noise levels—conditions that urban-dwelling birds would experience. Since birds might disperse away from the source of noise, we also investigated how the spatial distribution of the birds changed across a noise gradient. We created an experimental sound gradient of traffic noise across aviaries in which the amplitude of the noise varied from high noise (~ 80 dBA SPL) to lower noise (~ 70 dBA SPL) within a free-flight aviary (Fig. 1). Using flocks of captive-bred zebra finches (Taeniopygia guttata), we measured changes in individual social connectivity and whole-flock clustering before, during, and after introducing traffic noise. We also recorded how birds used the space across the sound gradient to determine if birds preferred using the quieter side of the aviary during the noise playback.
Zebra finches maintain their highly social flocking nature when housed in free-flight aviaries (Zann 1996). They quickly form pair bonds within the flock and are rarely without their social partner. Birds engage in flock activities such as feeding, watering, and allopreening (Zann 1996). Zebra finches rely on auditory cues for individual recognition (Zann 1996), making their social system particularly vulnerable to changes in noise conditions. Previous work on this species has demonstrated that noise inhibits cognitive performance and social learning (Osbrink et al. 2021), changes vigilance behavior while foraging (Evans et al. 2018), and decreases pair bond preferences (Swaddle and Page 2007b), which suggests larger changes could be occurring at the social network level in response to noise disturbance.
We hypothesized birds would change how they used aviary space in response to noise and predicted that they would increase time spent in the quieter end of the noise gradient at higher densities than before the noise treatment. We therefore hypothesized increased densities of conspecifics would affect individual social connectivity and whole-flock clustering predicting an increase in both connectivity and clustering with the onset of noise.
Methods
We used a large colony of outbred, domesticated zebra finches that were housed in outdoor aviaries at William & Mary (Williamsburg, Virginia, USA, 37.3° N, 76.7° W). Zebra finches used in this study were raised in relatively noisy conditions, as their parents had been for at least eight generations, due to the incidental effects of having a captive breeding colony in indoor rooms with a relatively old air conditioning system and highly reflective concrete walls and floors. Typical noise levels in indoor breeding rooms were between 55 and 86 dBA SPL, which varies based on air conditioner ventilation bouts and fluctuations in group vocalization among the colony. All procedures used on animals were approved by the Institutional Animal Care and Use Committee at William & Mary (IACUC-2015-11-16-10733-jpswad).
We conducted a total of 11 trials between May and October 2017 in two large outdoor aviaries. Each aviary consisted of three conjoined rooms (3 × 3 × 6 m each, a collective size of 9 × 3 × 6 m) among which birds could freely move (Fig. 1). Birds had ad libitum access to a nutritionally balanced diet of millet mix (Volkman Super Finch Blend), water, and cuttlefish bone. Feeders were placed in rooms 1 and 3 of each aviary (Fig. 1). For each trial, one of the two aviaries received a high-amplitude treatment where either room 1 or 3 had a speaker play traffic noise, creating an amplitude gradient across the three rooms where the furthest room from the speaker was the quietest. This allowed the birds to reduce their noise exposure, but not entirely escape it, which is ecologically relevant to urbanized areas. The noise continued across our facilities to the second aviary at a lower amplitude, with the loudest of the three rooms ~ 20 dBA quieter than the loudest room in the high-noise treatment; this aviary served as our “lower” noise treatment. The lower noise treatment only had a small increase in sound pressure (1–2 dB SPL) during playback from the high-noise treatment, as the traffic sounds were quiet relative to the background noise of the facility. However, the representation of traffic sounds was palpable even at this distance and novel to the lower noise birds, as the aviary is not located near any roads.
Plan view of aviary. Thick gray bars indicate wooden perches. The three cages were connected by one long perch, and central walls were covered in noise-absorbing foam. A 1-m-long high perch and a 0.5-m-long low perch were placed in rooms 1 (R1) and 3 (R3). F indicates food and water station (0.5-m diameter tray), and S indicates speakers (speakers not drawn to scale). We had two identical aviaries with this set-up to serve as high-amplitude noise and low-amplitude noise treatments, we alternated treatments throughout trials. For the high-noise treatment, the two speakers in either R1 or R3 (alternating between trials) would play traffic noise and the two speakers in the opposite room would remain inactive, creating a sound gradient across this structure
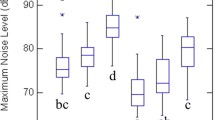
We took sound pressure readings using an EXTECH General Purpose Sound Level Meter and stood at the feeder locations in rooms 1 and 3 (Fig. 1) on day 2 of each experimental phase for each trial. The sound meter was calibrated with an EXTECH 407744 Sound Level Calibrator, which calibrates the meter with a 1 kHz sine wave at 94 dB SPL with an accuracy of ± 0.8 dB standard error. With no noise playing, peak amplitude of ambient noise pressure in the outdoor aviaries averaged 60.62 ± 0.52 standard error dBA SPL; this average was calculated by using all measurements from rooms 1 and 3 in both high- and lower-amplitude treatments from the before and after phases when there was no noise playback (n = 85). In the high-amplitude aviary during traffic noise playback, peak amplitude in the room with noise playback averaged 80.09 dBA SPL ± 0.99 SE and 70.94 dBA SPL ± 2.94 SE in the room furthest from the playback speaker (n = 11). In the lower-amplitude aviary during the traffic noise playback, peak amplitude averaged 62.53 dBA SPL ± 0.75 SE in the room closest to the high-amplitude aviary and 61.96 dBA SPL ± 0.98 SE in the furthest room (n = 11).
We used a pseudo-randomized blocking procedure to determine the aviary and room that would receive sound treatment; thus, we used both aviaries for high noise and low noise equally and alternated which room (1 or 3) had noise for each trial. This was to account for any preexisting preferences for rooms or aviaries that the birds may have, independent of our experimental manipulation.
For each trial, we released 12 birds (six adult male, six adult female) marked with individual color bands into the high-amplitude aviary and a separate 12 birds in the lower-amplitude aviary and gave them 72 h to habituate to the enclosure and establish their social groups with no noise playback. Of the 12 birds in each aviary, males and females were unfamiliar with each other, but birds could have been familiar with each other within the sexes, as individuals were previously housed with approximately 100 others in same-sex free-flight conditions. All birds were wild-type coloration (to the human eye) to control for any social preferences for unique colorations sometimes found in captivity (e.g., fawn, black-cheeked, white). Each bird was used only once and did not appear in subsequent trials. Hence, we used 264 birds over the course of 11 trials (24 birds per trial).
Following the initial 72-h habituation period, each trial consisted of three additional 72-h experimental phases: (i) “before” which was the phase before the experimental noise manipulation, (ii) “noise” when the high-amplitude aviary received traffic noise playback (which slightly reached our lower-amplitude aviary), and (iii) “after” which was the time period immediately following the termination of the active playback in the noise phase (Table 3). During the noise phase, speakers directed towards the opposite wall of their placement played a looped audio recording of highway traffic noise that varied in maximum dBA SPL (~ 65–80 dBA) depending on the number of cars and trucks passing on the highway throughout the recording (YouTube 2014) and played in either room 1 or 3 of the aviary depending on trial (Fig. 1). We had four speakers in each aviary set up (two in each of the far rooms) but only one amplifier set to a consistent volume that we moved between aviaries/rooms depending on which was getting the noise treatment in that trial to ensure consistency.
Observation periods took place at either 9:00 EST or 12:00 EST and would last for roughly 2 h (1 h and 36 min of focal-follow time), with time in between as observers searched for the next focal-follow bird. We alternated which aviary had the morning (9 a.m.) or afternoon (12 p.m.) observation daily, so at the end of the experiment, each aviary had an equal number of morning and afternoon observation periods. Speakers were turned on at the conclusion of the 72-h before phase (~ 14:00 EST), played traffic noise continuously day and night, and then turned off at the conclusion of the 72-h noise phase.
We conducted 8-min focal-follows of each bird and defined interactions with an ethogram (Table 1) to collect behavioral data. Social interactions for the social network were defined by proximity (0.5 m horizontal distance). We chose this distance as our shortest perches were 0.5 m, as was the food/water tray available in rooms 1 and 3 of the aviary set-up (Fig. 1). Each data collection period consisted of focal-follows of every bird in a randomized order in both treatments (experimental and reference) daily throughout a trial. During a focal-follow, one focal bird would be observed for 8 min, and only interactions with that focal bird were recorded. This process was repeated for each of the 12 birds in each data collection period. We had three different observers for data collection periods (only one observer was present at each data collection period), and these observers trained together on a pilot flock to determine whether observer bias existed. Using the pilot flock, the three observers that were present together focal-followed the same individuals at the same time to record interactions. Observers compared our recorded interactions, calculated social network metrics, and found minimal differences among our datasets. We confirmed no significant effect of observer using intraclass correlation coefficients. The three observers also rotated which flock they were observing every data collection period, so all three observed an equal number of high-noise or low-noise flocks across the two different aviaries and across experimental phases. Over the course of the study, 260 birds were observed for a total of 312 h of data collection (total birds had been 264, but four birds died early in their trials and were thus excluded from the data).
We constructed social networks using proximity data, and an interaction is defined as an individual within 0.5 m horizontally of other individuals (e.g., birds were on the same perch or using the same resource), but only with non-aggressive sub-behaviors (shown in white, Table 1), as aggressive sub-behaviors (shown in gray, Table 1) tended to be the displacement of another individual to keep them away from their social group. The detailed non-aggressive sub-behaviors described in our ethogram were for a different project; for our purposes, we were interested in the animals as a group, and so our intent was not to separate these sub-behaviors when constructing our social network.
We then transformed interaction data into association matrices using the simple ratio index (SRI): \(\frac{x}{x+{y}_{a}+{y}_{b}+{y}_{ab}}\) where x is the total number of observations where “a” and “b” are associated, ya is the number of times the focal individual (a) was observed, yb is the number of times the interacting individual (b) was observed, and yab is the total observations where the focal individual and interacting individual were each observed, but not together. An individual’s total number of observations (ya and yb) were calculated by including any appearance of the individual in a focal-follow recording (either as an interacting bird or during its own focal-follow) and summed once focal-follows were completed for all 12 birds. The SRI value represents the strength of the relationship between individuals (or the “edges” between the “nodes” in a social network), weighted by the frequency of interactions. All SRI values are from 0 to 1, 0 being the two individuals never interacted and 1 being that every time individual “a” is observed, “b” is interacting with it. An individual’s sociality can be measured using weighted degree, which is the sum of all SRI values associated with that individual (or the sum of all edge values attached to the node in a social network). Thus, weighted degree accounts for both the total number of social partners that individual has and the frequency of interactions with those social partners.
At the group level, we measured global clustering coefficient which measures how connected the overall social network is by summing the number of closed triplets (a group of 3 birds all connected to each other) divided by the number of triplets of birds that are connected to each other in some way (either as a closed triangle or an open triplet with one or two edges) (Whitehead 2008). This generates a proportion between 0 and 1, where 0 is no three birds are all connected to each other, and 1 is every possible triplet in the network is closed. Weighted degree and global clustering coefficient are repeatable and consistent across time in other studies that lack an experimental intervention (Jacoby et al. 2014), making them suitable candidate metrics to study the effects of noise disturbance on individual and group sociality, as any observed changes in these metrics could be attributed to our noise treatment. We calculated individual weighted degree and flock global clustering coefficient at the end of each experimental phase (72-h period; Before, Noise, and After).
To determine space use, we used a digital data collection sheet that logged time stamps for each entry. A time stamp was recorded at the start of the trial, and additional time stamps would record the exact time a bird switched rooms. These time stamps allowed us to determine the total number of seconds a focal bird spent in each of the three rooms in an aviary. We then calculated the relative proportion of time spent in each room for the focal bird for each phase (Before, Noise, After) by calculating the total seconds spent in each room and dividing this by 1440 s (total 24 min) because each individual had three 8-min focal-follow periods for each experimental phase. This resulted in a percentage value representative of the relative amount of time a bird spent in each room throughout the experiment. For our statistical analysis, we only included the proportion of time spent in the room that had the speakers playing in the Noise phase in the high-noise aviary. For the low-noise aviary, we did this same calculation for the room in closest physical proximity to the high-noise aviary.
Statistical analyses
We wrote a custom Python script to manipulate data which converted raw interaction data to a matrix of association indices. To process social network metrics, we used the “igraph” package in R (Csárdi and Nepusz 2006; R Core Team 2016). To analyze spatial use of the aviary with the onset of noise in our high-noise treatment, we calculated the difference in the proportion of time birds spent in the room with active playback from before to noise periods for the high-noise and low-noise treatments. For the lower noise treatment, we did this calculation for the room in closest proximity to the high-noise aviary. We used generalized linear mixed models (GLMMs) for each of our response variables, weighted degree (gamma model), GCC (Gaussian model), and space use (zero-inflated beta model) using the “lme4” package in R (Bates et al. 2015; R Core Team 2016). Our fixed effects were Treatment which had two levels (low noise and high noise), Phase, which had three levels (before, noise, after), and their interaction. Individual bird identity nested within trial was a random effect for all three models. For GCC, we used a logit transformation to fit a normal distribution, as values were proportions bound between 0 and 1. We obtained p-values for predictors using Wald tests.
Sampling bias
We recognize the risk of sampling bias when collecting social network data and did our best to mitigate potential risks. For example, an observer misidentifying an individual could generate a social network that fails to reveal the actual social structure, particularly with global metrics like global clustering coefficient (Davis et al. 2018). Therefore, if an observer was unsure about the identity of an individual, they were trained to skip this social interaction rather than attempt to guess at the individual’s identity, as missed interactions have less impact on overall network structure than misidentified interactions (Davis et al. 2018). It is worth noting that local metrics, such as weighted degree used in this study, are more robust to observer error. All observers in this study trained on a pilot flock of birds to practice taking observations before the experiment began, and we feel confident in their ability to accurately re-sight color bands to record proximity interactions.
Results
Changes to individual sociality (weighted degree)
Sociality was significantly influenced by Treatment (p < 0.001), phase (p < 0.001), and their interaction (p < 0.001). The effect of Treatment (β1 = 0.19656 ± S.E. = 0.070) indicates that on average, weighted degree increased more in the high-noise treatment compared to the low-noise treatment. The Noise phase increased weighted degree of birds compared to the Before phase (Noise, β2 = 0.12364 ± 0.058) meaning birds had on average more social partners and more interactions with their social partners during Noise in both treatments. Following the termination of the noise treatment in the After phase, weighted degree decreased to levels below, but not significantly different from, that of their original state in the Before phase (After, β3 = − 0.10323 ± 0.053). These effects, as well as the interaction effects, are given in Table 2. These results reveal that birds experienced change in sociality directly related to the Noise phase of the experiment and returned to a similar social state as Before following the termination of noise in the After phase (Fig. 2).
Changes to flock (global clustering coefficient)
Global clustering coefficient (GCC), or overall connectedness of the network, was significantly influenced by Phase (F2,40, p = 0.013), but not by Treatment (F1,20, p = 0.054) or by their interaction (F2,40, p = 0.717). Individual factors on their own were not statistically significant, but the overall impact of Phase did demonstrate changes in clustering patterns associated with the onset of the Noise treatment (Table 3, Fig. 3).
Changes to space use
Our zero-inflated beta model results in two models, a conditional model that predicts the actual non-zero value of the proportion of time spent in the room of interest and a zero-inflation model that predicts the likelihood of observing a zero in a particular condition (Treatment and/or Phase). In biological context, a zero occurs when no individuals use the room of interest during the entire observation period. Our conditional model demonstrated that changes in space use for the room that received noise treatment were significantly influenced by the interaction of Treatment and Phase (F2,474, p = 0.001) (Table 4). Additionally, zero-values were more likely to occur in the After phase or when considering the interaction between Noise*Treatment and After*Treatment (Table 5). Post hoc paired Wilcoxon rank-sum tests reveal that the significant interaction results in the conditional model were driven by the change between Before and Noise and, interestingly, in opposite directions dependent on treatment. Birds in the high-noise group increased their use of the room that received noise (p < 0.001), and birds in the low-noise treatment decreased their use of the room closest to the noise (p < 0.01) (Fig. 4). This differential response (positive response in high noise, negative response in low noise) led to our mixed model failing to detect the effect of Phase alone (F2,474, p = 0.956), as the mean difference was averaged between the two.
Changes in the average proportion of time spent in the room that received noise treatment (high noise) or the room closest adjacent to the noise treatment (low noise) during the Noise phase throughout the trials for high-noise birds (n = 131) and low-noise birds (n = 129) with corresponding ± one standard error bars (raw data)
Discussion
We found that individual sociality (weighted degree) and flock clustering (GCC) both increase with the onset of introduced traffic noise, although the mechanism driving these social changes remains to be discovered. We also observed that removing the noise source led to the birds becoming less social (decrease in weighted degree) than in their pre-disturbed state. Changes in social behavior occurred in both treatment groups (high and lower amplitude), revealing that even in low levels of traffic noise, birds adjusted their social behavior following some exposure to traffic noise sounds. However, the high-amplitude treatment birds experienced a more pronounced response in weighted degree compared to birds in the lower-amplitude groups. We recognize that a no-noise condition would have been ideal for this experiment, but this was not feasible with our aviary resources, as the proximity of aviaries was such that we could not isolate the traffic noise sounds to the high-amplitude playback only. Therefore, we are not able to reject the possibility that the observed pattern is a result of the flock’s social progression over time; however, given the significantly steeper rate of change in the high-amplitude conditions (Figs. 2 and 3), we can conclude that the amplitude of traffic noise exposure playback affected social behavior.
Although we initially predicted that observed changes in social behavior would be driven by changes in space use, we observed conflicting results in changes in space use that were dependent on noise treatment. Unexpectedly, birds that received a high-amplitude treatment spent a higher proportion of time in the playback room during noise than before playback. Birds that received the lower-amplitude treatment decreased their use of the room in closest physical proximity to the high-noise treatment with the onset of noise, which was more in line with our initial predictions. Because our social responses (weighted degree and GCC) between treatment groups were positively correlated with experimental phase in both treatments, but we observed a divergent response dependent on treatment, we propose that changes in social behavior must be driven by alternative mechanisms.
In free-living conditions without space constraints, the densities of birds often decrease in areas with introduced experimental noise playback (Francis et al. 2009; McClure et al. 2013; Mahjoub et al. 2015; Swaddle et al. 2016; Gomes et al. 2021). However, this response is species-dependent with species such as house finches (Carpodacus mexicanus) and black-chinned hummingbirds (Archilochus alexandri) increasing densities in noise sites (possibly due to the dispersal of nest predators) (Francis et al. 2009) or not demonstrating changes in their distributions in response to noise at all, as is the case with red-backed fairywrens (Hawkins et al. 2020). Our study was spatially constrained, with captive birds unable to fully escape noise playback.
In a similar captive study with domesticated zebra finches, birds spent less time in the highest amplitude space during acute playbacks of traffic noise (30 min) (Liu et al. 2020). The birds in our study were exposed to chronic traffic noise over 72 h, and the first measurement of changes in space use occurred after approximately 20-h habituation period to the onset of noise. As social interactions and relationships develop over longer time intervals, we therefore suggest that changes in space use are not the most likely mechanism for changes in social behavior in response to noise.
Despite the known interference of noise with communication regarding signal transmission, we observed increased social behavior in high-amplitude and lower-amplitude conditions. Potential mechanisms for increased flock clustering and individual sociality could be increased perceived predation risk (Mahjoub et al. 2015) as noise decreases detection ability for low-frequency predator cues (Jung et al. 2020), and thus, increased flock density could serve as an antipredatory strategy (Peacor and Lansing 2003; Owens et al. 2012). Indeed, increased vigilance behavior in experimental noise playback has been documented in mammals, birds, and fish (Voellmy et al. 2014; Meillere et al. 2015; Kern and Radford 2016). Free-living dwarf mongoose (Helogale parvula) and captive white-crowned sparrows (Zonotrichia leucophrys) decreased feeding duration and increased vigilance decreased time spent foraging and increased time spent scanning or vigilant (Ware et al. 2015; Kern and Radford 2016). Song sparrows (Melospiza melodia) increased vigilance or decreased foraging in playbacks of high noise from both natural (rivers) and anthropogenic (road) sources (Sweet et al. 2022). Female house sparrows had shorter flushing distances when researchers approached nest boxes in areas with traffic noise playback (Meillere et al. 2015). Three-spined sticklebacks reacted more quickly to a visual predator cue during playback of boat noise (Voellmy et al. 2014). Across avian species, foraging and non-foraging group sizes decrease with relaxed predation risk (Beauchamp 2010), so our result of increased social connectivity in response to increased predation risk is consistent with this pattern.
Alternatively, or in addition to increased perceived predation risk, it is possible that the increased social response is acting as a social buffer to a stressor. Research on the effects of noise on glucocorticoid profiles of birds has yielded mixed results. For baseline corticosterone, house wrens from rural areas exposed to noise had elevated CORT and their urban counterparts did not, but captive zebra finches exposed to noise had lower CORT than their no-noise counterparts (Davies et al. 2017; Zollinger et al. 2019). Nestling, but not adult, tree swallows (Tachycineta bicolor) exposed to noise in field conditions also had elevated baseline CORT, but nestling mountain white-crowned sparrows had depressed baseline CORT (Crino et al. 2013; Injaian et al. 2018a). However, in nestling house sparrows, there was no apparent effect of exposed traffic noise to baseline CORT or stress response (Angelier et al. 2016). Although the impacts of noise exposure on the glucocorticoid profiles of birds remain subject to further investigation, one explanation for our observed increased social behavior could be social buffering against changes to glucocorticoids. For example, in zebra finches, birds exposed to a mild stressor had reduced stress response if they were in the presence of a familiar conspecific (Emmerson and Spencer 2017). Additionally, we speculate that the observed increase of space use in the noise room for birds in the high-noise treatment could have been driven by a stress-freezing behavior (Korte et al. 2005; De Haas et al. 2012). We observed many of the birds in the high-noise treatment shifted to spending 100% of their time in the noise room with the onset of noise. We recommend further research on how social buffering can possibly mediate the negative effects of noise and how stress response to noise can affect spatial decisions.
As noise is not limited to acoustic interference of communication signals, cross-sensory interference could be another potential mechanism for changes in social behavior (Chan et al. 2010). For example, animals exhibit a dampened response to visual cues in high-noise conditions, such as fewer cryptic prey located by great tits (Parus major) (Halfwerk and Van Oers 2020) and reduced response to attractive visual cues of male serrate-legged small treefrogs (Kurixalus odontotarsus) by females (Zhu et al. 2022). In high-noise environments, dwarf mongooses had delayed response to olfactory cues of predator feces (Morris-Drake et al. 2016). Social behavior is inherently a cross-sensory process (e.g., visual signals like baring teeth in primates, olfactory cues like scent marking in canids and felids, acoustic signals like duetting in songbirds), so there are many avenues in which noise could affect social interactions that are worth exploring in future studies.
Increased social behavior in urban-dwelling birds can have a variety of ecological implications for urban sedentary species. Both social network size and individual sociality affect disease transmission, as individuals with a high weighted degree have a disproportionate effect on the disease transmission throughout a population (Hamede et al. 2009; Rushmore et al. 2013). Thus, denser flocks of birds with more social constituents could alter the rate and prevalence of pathogen transmission. Urban-dwelling birds tend to have increased parasite loads compared to their rural counterparts, and increased social behavior potentially contributes to this pattern (Giraudeau et al. 2014). Possible benefits of increased social behavior include increased transmission of symbiotic microbes that change the gut microflora biodiversity in birds, increasing resistance to disease (Archie and Tung 2015; Levin et al. 2016). Additionally, denser group clustering can increase familiarity between conspecifics, which can decrease individual aggressiveness (Geffroy et al. 2014).
The reduction in social behavior following disturbance in the After phase indicates a level of resistance to disturbance in domesticated zebra finches. While the resulting decreased levels of weighted degree surpassed their baseline levels in the Before phase, it would be relevant to extend the timeline of observations to see if this would return to baseline after more time. Recovering from a disturbed social network implies that remediation strategies for noise pollution could return birds to their natural social state, a phenomenon observed in bottlenose dolphin (Tursiops aduncus) social networks following the implementation of legislation limiting commercial fisheries in their habitat (Ansmann et al. 2012).
Due to the highly social nature of our study system—zebra finches in the wild often live in a range of flock sizes, either smaller or larger than the one we have created here (Zann 1996; McCowan et al. 2015)—our results may not be generalizable to avian species that have different social systems (e.g., solitary birds). Indeed, previous research on a free-living population of red-backed fairywrens, a passerine that forms small familial groups, found that weighted degree decreased with the onset of experimental noise (Hawkins et al. 2020). Additionally, the challenges that free-living birds face, such as predation risk or access to resources, would likely result in selection pressures on populations that influence social dynamics that our captive population did not experience. Notably, previous research on the effects of early-life stress on social network position has demonstrated consistent results between zebra finches in captive studies and studies of wild populations (Brandl et al. 2019). Despite limitations inherent in a captive environment, we feel our results are relevant to interpreting the effects of noise on other highly social species that reside in urban areas in which anthropogenic landscapes fragment habitat and variable gradients of traffic noise are pervasive (e.g., great tits, rock doves (Columba livia), barnacle geese (Branta leucopsis), and gallahs (Eolophus roseicapilla)).
In conclusion, our study provides evidence that traffic noise alters individual sociality and flock clustering in a population chronically exposed to anthropogenic noise. We suggest that the observed changes are driven by factors other than noise-induced constraints on space use and propose hypotheses related to perceived predation risk, social buffering to stress, vigilance, and cross-sensory interference. The rebound of social behavior following the noise introduction suggests that remediation strategies for reducing noise pollution in urban areas stand to benefit urban-dwelling species.
Data availability
The audio file for playback, datasets, and code for the statistical analyses described in this manuscript are provided here https://zenodo.org/doi/10.5281/zenodo.7869070.
References
Angelier F, Meillère A, Grace JK et al (2016) No evidence for an effect of traffic noise on the development of the corticosterone stress response in an urban exploiter. Gen Comp Endocrinol 232:43–50. https://doi.org/10.1016/j.ygcen.2015.12.007
Ansmann IC, Parra GJ, Chilvers BL, Lanyon JM (2012) Dolphins restructure social system after reduction of commercial fisheries. Anim Behav 84:575–581. https://doi.org/10.1016/j.anbehav.2012.06.009
Archie EA, Tung J (2015) Social behavior and the microbiome. Curr Opin Behav Sci 6:28–34. https://doi.org/10.1016/j.cobeha.2015.07.008
Balasubramaniam KN, Kaburu SSK, Marty PR et al (2021) Implementing social network analysis to understand the socioecology of wildlife co-occurrence and joint interactions with humans in anthropogenic environments. J Anim Ecol 90:2819–2833. https://doi.org/10.1111/1365-2656.13584
Baldassarre DT, Greig EI, Webster MS et al (2016) The couple that sings together stays together: duetting, aggression and extrapair paternity in a promiscuous bird species. Biol Lett 12:1–4. https://doi.org/10.1098/rsbl.2015.1025
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Beauchamp G (2010) Relaxed predation risk reduces but does not eliminate sociality in birds. Biol Lett 6:472–474. https://doi.org/10.1098/rsbl.2009.1063
Brandl HB, Farine DR, Funghi C et al (2019) Early-life social environment predicts social network position in wild zebra finches. Proc R Soc B 286:20182579. https://doi.org/10.1098/rspb.2018.2579
Bruintjes R, Radford AN (2013) Context-dependent impacts of anthropogenic noise on individual and social behaviour in a cooperatively breeding fish. Anim Behav 85:1343–1349. https://doi.org/10.1016/j.anbehav.2013.03.025
Brumm H (2004) The impact of environmental noise on song amplitude in a territorial bird. J An Ecol 73:434–440
Brumm H, Slabbekoorn H (2005) Acoustic communication in noise. Adv Study Behav 35:151–209. https://doi.org/10.1016/S0065-3454(05)35004-2
Cartwright LA, Taylor DR, Wilson DR, Chow-Fraser P (2014) Urban noise affects song structure and daily patterns of song production in red-winged blackbirds (Agelaius phoeniceus). Urban Ecosyst 17:561–572. https://doi.org/10.1007/s11252-013-0318-z
Chan AAYH, Giraldo-Perez P, Smith S, Blumstein DT (2010) Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis. Biol Lett 6:458–461. https://doi.org/10.1098/rsbl.2009.1081
Chen HL, Koprowski JL (2015) Animal occurrence and space use change in the landscape of anthropogenic noise. Biol Conserv 192:315–322. https://doi.org/10.1016/j.biocon.2015.10.003
Crino OL, Johnson EE, Blickley JL et al (2013) Effects of experimentally elevated traffic noise on nestling white-crowned sparrow stress physiology, immune function and life history. J Exp Biol 216:2055–2062. https://doi.org/10.1242/jeb.081109
Csárdi G, Nepusz T (2006) The igraph software package for complex network research. Int J Complex Syst 1695:1–9. https://doi.org/10.3724/SP.J.1087.2009.02191
Davies S, Haddad N, Ouyang JQ (2017) Stressful city sounds: glucocorticoid responses to experimental traffic noise are environmentally dependent. Biol Lett 13. https://doi.org/10.1098/rsbl.2017.0276
Davis GH, Crofoot MC, Farine DR (2018) Estimating the robustness and uncertainty of animal social networks using different observational methods. Anim Behav 141:29–44. https://doi.org/10.1016/j.anbehav.2018.04.012
De Haas EN, Kops MS, Bolhuis JE et al (2012) The relation between fearfulness in young and stress-response in adult laying hens, on individual and group level. Physiol Behav 107:433–439. https://doi.org/10.1016/j.physbeh.2012.08.002
Derryberry EP, Phillips JN, Derryberry GE et al (1979) (2020) Singing in a silent spring: birds respond to a half-century soundscape reversion during the COVID-19 shutdown. Science 21:1–9. https://doi.org/10.1016/j.solener.2019.02.027
Derryberry EP, Danner RM, Danner JE et al (2016) Patterns of song across natural and anthropogenic soundscapes suggest that white-crowned sparrows minimize acoustic masking and maximize signal content. PLoS ONE 11:1–17. https://doi.org/10.1371/journal.pone.0154456
Derryberry EP, Gentry K, Derryberry GE et al (2017) White-crowned sparrow males show immediate flexibility in song amplitude but not in song minimum frequency in response to changes in noise levels in the field. Ecol Evol 7:4991–5001. https://doi.org/10.1002/ece3.3037
Emmerson MG, Spencer KA (2017) Long-term effects of adolescent stress on neophobic behaviors in zebra finches are modulated by social context when in adulthood. Horm Behav 90:48–55. https://doi.org/10.1016/j.yhbeh.2017.02.004
Evans JC, Dall SRX, Kight CR (2018) Effects of ambient noise on zebra finch vigilance and foraging efficiency. PLoS ONE 13:17–22. https://doi.org/10.1371/journal.pone.0209471
Francis CD, Ortega CP, Cruz A (2009) Noise pollution changes avian communities and species interactions. Curr Biol 19:1415–1419. https://doi.org/10.1016/j.cub.2009.06.052
Fuller RA, Warren PH, Gaston KJ (2007) Daytime noise predicts nocturnal singing in urban robins. Biol Lett 3:368–370. https://doi.org/10.1098/rsbl.2007.0134
Geffroy B, Bru N, Dossou-Gbété S et al (2014) The link between social network density and rank-order consistency of aggressiveness in juvenile eels. Behav Ecol Sociobiol 68:1073–1083. https://doi.org/10.1007/s00265-014-1719-6
Giraudeau M, Mousel M, Earl S, McGraw K (2014) Parasites in the city: degree of urbanization predicts poxvirus and coccidian infections in house finches (Haemorhous mexicanus). PLoS One 9. https://doi.org/10.1371/journal.pone.0086747
Gomes DGE, Toth CA, Cole HJ et al (2021) Phantom rivers filter birds and bats by acoustic niche. Nat Commun 12. https://doi.org/10.1038/s41467-021-22390-y
Grunst AS, Grunst ML, Pinxten R, Eens M (2019) Personality and plasticity in neophobia levels vary with anthropogenic disturbance but not toxic metal exposure in urban great tits: urban disturbance, metal pollution and neophobia. Sci Total Environ 656:997–1009. https://doi.org/10.1016/j.scitotenv.2018.11.383
Halfwerk W, Van Oers K (2020) Anthropogenic noise impairs foraging for cryptic prey via cross-sensory interference. Proc R Soc B Biol Sci 287. https://doi.org/10.1098/rspb.2019.2951
Hamede RK, Bashford J, McCallum H, Jones M (2009) Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population : using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecol Lett 12:1147–1157. https://doi.org/10.1111/j.1461-0248.2009.01370.x
Harding HR, Gordon TAC, Eastcott E et al (2019) Causes and consequences of intraspecific variation in animal responses to anthropogenic noise. Behav Ecol 30:1501–1511. https://doi.org/10.1093/beheco/arz114
Hawkins CE, Ritrovato IT, Swaddle JP (2020) Traffic noise alters individual social connectivity, but not space-use, of red-backed fairywrens. Emu - Austral Ornithology 313–321. https://doi.org/10.1080/01584197.2020.1830706
Injaian AS, Poon LY, Patricelli GL (2018a) Effects of experimental anthropogenic noise on avian settlement patterns and reproductive success. Behav Ecol 29:1181–1189
Injaian AS, Taff CC, Pearson KL et al (2018b) Effects of experimental chronic traffic noise exposure on adult and nestling corticosterone levels, and nestling body condition in a free-living bird. Horm Behav 106:19–27. https://doi.org/10.1016/j.yhbeh.2018.07.012
Jacoby DMP, Fear LN, Sims DW, Croft DP (2014) Shark personalities? Repeatability of social network traits in a widely distributed predatory fish. Behav Ecol Sociobiol 68:1995–2003. https://doi.org/10.1007/s00265-014-1805-9
Jung H, Sherrod A, LeBreux S et al (2020) Traffic noise and responses to a simulated approaching avian predator in mixed-species flocks of chickadees, titmice, and nuthatches. Ethology 126:620–629. https://doi.org/10.1111/eth.13013
Kern JM, Radford AN (2016) Anthropogenic noise disrupts use of vocal information about predation risk. Environ Pollut 218:988–995. https://doi.org/10.1016/j.envpol.2016.08.049
Kight CR, Swaddle JP (2015) Eastern bluebirds alter their song in response to anthropogenic changes in the acoustic environment. In: Integrative and Comparative Biology
Korte SM, Koolhaas JM, Wingfield JC, McEwen BS (2005) The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev 29:3–38. https://doi.org/10.1016/j.neubiorev.2004.08.009
Kunc HP, Lyons GN, Sigwart JD et al (2014) Anthropogenic noise affects behavior across sensory modalities. Am Nat 184:E93–E100. https://doi.org/10.1086/677545
Levin II, Zonana DM, Fosdick BM et al (2016) Stress response, gut microbial diversity and sexual signals correlate with social interactions. Biol Lett 12:20160352
Liu Q, Slabbekoorn H, Riebel K (2020) Zebra finches show spatial avoidance of near but not far distance traffic noise. Behaviour 157:1–30. https://doi.org/10.1163/1568539X-bja10004
Luther DA, Phillips J, Derryberry EP (2016) Not so sexy in the city: urban birds adjust songs to noise but compromise vocal performance. Behav Ecol 27:332–340. https://doi.org/10.1093/beheco/arv162
Mahjoub G, Hinders MK, Swaddle JP (2015) Using a “sonic net” to deter pest bird species: excluding European starlings from food sources by disrupting their acoustic communication. Wildl Soc Bull 39:326–333. https://doi.org/10.1002/wsb.529
McClure CJW, Ware HE, Carlisle J et al (2013) An experimental investigation into the effects of traffic noise on distributions of birds: avoiding the phantom road. Proc Royal Soc B 280:20132290. https://doi.org/10.1098/rspb.2013.2290
McCowan LSC, Mariette MM, Griffith SC (2015) The size and composition of social groups in the wild zebra finch. Emu 115:191–198. https://doi.org/10.1071/MU14059
McMullen H, Schmidt R, Kunc HP (2014) Anthropogenic noise affects vocal interactions. Behav Proc 103:125–128. https://doi.org/10.1016/j.beproc.2013.12.001
Meillere A, Brischoux F, Angelier F (2015) Impact of chronic noise exposure on antipredator behavior: an experiment in breeding house sparrows. Behav Ecol 26:569–577. https://doi.org/10.1093/beheco/aru232
Meise K, Franks DW, Bro-Jørgensen J (2019) Using social network analysis of mixed species groups in African savannah herbivores to assess how community structure responds to environmental change. Philos Trans R Soc B Biol Sci 374. https://doi.org/10.1098/rstb.2019.0009
Morris-Drake A, Kern JM, Radford AN (2016) Cross-modal impacts of anthropogenic noise on information use. Curr Biol 26:R911–R912. https://doi.org/10.1016/j.cub.2016.08.064
Nowicki S, Searcy WA, Hughes M (1998) The territory defense function of song in song sparrows : a test with the speaker occupation. Brill 135:615–628
Osbrink A, Meatte MA, Tran A et al (2021) Traffic noise inhibits cognitive performance in a songbird. Proc R Soc B Biol Sci 288. https://doi.org/10.1098/rspb.2020.2851
Owens JL, Stec CL, O’Hatnick A (2012) The effects of extended exposure to traffic noise on parid social and risk-taking behavior. Behav Proc 91:61–69. https://doi.org/10.1016/j.beproc.2012.05.010
Peacor SD, Lansing E (2003) Phenotypic modifications to conspecific density arising from predation risk assessment. Nordic Society Oikos 100:409–415
Poesel A, Kunc HP, Foerster K et al (2006) Early birds are sexy: male age, dawn song and extrapair paternity in blue tits, Cyanistes (formerly Parus) caeruleus. Anim Behav 72:531–538. https://doi.org/10.1016/j.anbehav.2005.10.022
R Core Team (2016) R: a language and environment for statistical computing
Rushmore J, Caillaud D, Matamba L et al (2013) Social network analysis of wild chimpanzees provides insights for predicting infectious disease risk. J Anim Ecol 82:976–986. https://doi.org/10.1111/1365-2656.12088
Sih A, Trimmer PC, Ehlman SM (2016) A conceptual framework for understanding behavioral responses to HIREC. Curr Opin Behav Sci 12:109–114. https://doi.org/10.1016/j.cobeha.2016.09.014
Swaddle JP, Page LC (2007a) High levels of environmental noise erode pair preferences in zebra finches: implications for noise pollution. Anim Behav 74:363–368. https://doi.org/10.1016/j.anbehav.2007.01.004
Swaddle JP, Page LC (2007b) High levels of environmental noise erode pair preferences in zebra finches: implications for noise pollution. Anim Behav. https://doi.org/10.1016/j.anbehav.2007.01.004
Swaddle JP, Moseley DL, Hinders MK, Elizabeth SP (2016) A sonic net excludes birds from an airfield: implications for reducing bird strike and crop losses. Ecol Appl 26:339–345. https://doi.org/10.1890/07-1650.1
Sweet KA, Sweet BP, Gomes DGE et al (2022) Natural and anthropogenic noise increase vigilance and decrease foraging behaviors in song sparrows. Behav Ecol 33:288–297. https://doi.org/10.1093/beheco/arab141
Urban R, Jones G, Furmankiewicz J, Ruczyn I (2011) Social calls provide tree-dwelling bats with information about the location of conspecifics at roosts. Ethology 117:480–489. https://doi.org/10.1111/j.1439-0310.2011.01897.x
Voellmy IK, Purser J, Simpson SD, Radford AN (2014) Increased noise levels have different impacts on the anti-predator behaviour of two sympatric fish species. PLoS ONE 9:1–8. https://doi.org/10.1371/journal.pone.0102946
Wale MA, Simpson SD, Radford AN (2013) Noise negatively affects foraging and antipredator behaviour in shore crabs. Anim Behav 86:111–118. https://doi.org/10.1016/j.anbehav.2013.05.001
Ware HE, McClure CJW, Carlisle JD, Barber JR (2015) A phantom road experiment reveals traffic noise is an invisible source of habitat degradation. Proc Natl Acad Sci USA 112:201504710. https://doi.org/10.1073/pnas.1504710112
Whitehead H (2008) Analyzing animal societies: quantitative methods for vertebrate social analysis. University of Chicago Press, Chicago
Wiśniewska M, Puga-Gonzalez I, Lee P et al (2022) Simulated poaching affects global connectivity and efficiency in social networks of African savanna elephants-an exemplar of how human disturbance impacts group-living species. PLoS Comput Biol 18:1–23. https://doi.org/10.1371/journal.pcbi.1009792
YouTube (2014) Highway sounds white noise, cars passing by on freeway sounds, 8 Hours of Traffic White Noise for Sleep.
Zann RA (1996) The zebra finch: a synthesis of field and laboratory studies. Oxford University Press Inc
Zhu B, Zhang H, Chen Q et al (2022) Noise affects mate choice based on visual information via cross-sensory interference. Environ Pollut 308:119680. https://doi.org/10.1016/j.envpol.2022.119680
Zollinger SA, Dorado-Correa A, Goymann W et al (2019) Traffic noise exposure depresses plasma corticosterone and delays offspring growth in breeding zebra finches. Conserv Physiol 7. https://doi.org/10.1093/conphys/coz056
Zwart MC, Dunn JC, McGowan PJK, Whittingham MJ (2016) Wind farm noise suppresses territorial defense behavior in a songbird. Behav Ecol 27:101–108. https://doi.org/10.1093/beheco/arv128
Acknowledgements
We thank Ananda Menon, Juan Botero, and Shirley Mitchell for their assistance with setting up and maintaining our aviaries and captive zebra finch population.
Funding
This material is based upon work supported by awards to C. E. Hawkins from the Virginia Space Grant Consortium, the Coastal Virginia Wildlife Observatory, the Williamsburg Bird Club, and the Arts & Science Graduate School at William & Mary. We are immensely grateful for their contributions to this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hawkins, C.E., Pantel, J.H., Palia, S.T. et al. Zebra finches increase social behavior in traffic noise: Implications for urban songbirds. acta ethol 27, 13–25 (2024). https://doi.org/10.1007/s10211-023-00434-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10211-023-00434-4