Abstract
Dark artificial surfaces reflecting highly and horizontally polarized light usually have negative effects on polarotactic aquatic insects detecting their habitats by the horizontal polarization of water-reflected light. This ecologically disadvantageous phenomenon is called polarized light pollution. We have observed that the water between the concrete walls of a harbour of the Hungarian Lake Balaton is continuously dark from autumn to spring due to the inflow of a canal rich in dissolved humic substances. Using ground-born imaging polarimetry, we demonstrated that this dark water patch reflects light with higher degrees of polarization than the brighter lake water. Our hypothesis was that the stronger horizontally polarized light reflected from the dark water patch is more attractive to swarming, water-seeking and egg-laying non-biting midges (Chironomidae) than the surrounding brighter lake water. With larval samplings, we showed that both the density and the average size of chironomid larvae were significantly larger in the harbour than in the surrounding lake. This finding may represent an ecological advantage of polarized light pollution: polarotactic chironomids are intensely attracted to a strongly and horizontally polarizing, seasonally dark water patch at the canal inflow, where the abundance of larvae increases. It should be taken into consideration that increased larval abundance might result in increased swarming intensity which could affect humans by causing considerable nuisance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The larvae of non-biting midges (Diptera, Chironomidae) play an important role in benthic ecosystems and often are abundant in freshwater aquatic communities (Cranston 1995). They feed on particulate organic matter, bacteria and algae, constituting the base of the aquatic food web. The conspicuous swarming of the short-living imagoes usually takes only a few days (Armitage 1995). During their mass swarming, the imagoes take a considerable amount of organic matter out of water bodies, which substantially decreases the nitrogen and phosphorus concentration. In some cases, the imago carcasses play a role in fertilizing agricultural lands. On the other hand, dense swarms of chironomids often serve as nuisances for humans living and enjoying recreational activities in areas close to breeding places (Ali 1980; Lin and Quek 2011). Just to mention a few examples, due to their positive phototaxis, swarming chironomids are often attracted to artificial outdoor and indoor light sources in urbanized areas causing discomfort for tourists and residents, or in extreme cases, the slippery layer of their carcasses on the surface of roads increases the risk of accidents (Ali 1980).
Many aquatic insect species are positively polarotactic and recognize their aquatic habitats by means of the horizontal polarization of water-reflected light (Schwind 1991, 1995; Horváth and Varjú 2004; Horváth 2014). Several chironomid species are also polarotactic (Lerner et al. 2008, 2011; Meltser et al. 2008; Horváth et al. 2011; Robertson et al. 2018).
Shiny and dark artificial surfaces reflecting highly and horizontally polarized light can act as a supernormal stimulus, that deceives and attracts polarotactic insects even if real water bodies are visible in the optical environment. When a water-seeking flying polarotactic aquatic insect has to choose between water and such a horizontally polarizing manmade surface, it usually selects the latter because of the reflected supernormal polarization stimulus (Horváth 1995, 2014; Horváth and Varjú 2004; Horváth and Kriska 2008). The revelation of this phenomenon has led to the term ‘polarized light pollution’ being a special form of ecological photopollution and traps (Horváth et al. 2009). Typical sources of polarized light pollution are solar panels, glass buildings, black car bodies and asphalt roads, for example (Horváth et al. 2009). Several studies demonstrated the negative effects of polarized light pollution on a variety of aquatic insects including mayflies, caddisflies, dragonflies and tabanids, for instance (Kriska et al. 1998, 2008, 2009; Egri et al. 2017). These insects tend to oviposit on artificial surfaces reflecting strongly and horizontally polarized light; thus, the risk of their reproductive failure greatly increases (Kriska et al. 1998). One of the most drastic examples for polarized light pollution has been described by Horváth and Zeil (1996), who demonstrated how black oil lakes in the desert of Kuwait attract and kill large numbers of water-seeking polarotactic aquatic insects.
In some special cases, the water body has an isolated area and is much darker than the surrounding water. We observed such a canal carrying dark organic matter into the Hungarian Lake Balaton where chironomids are abundant (Fig. 1a, b, Electronic Supplementary Material M1–3). If the canal directly flows into a harbour, the intensity contrast between the darker and the surrounding brighter water can be extremely high (Fig. 1c–f, Electronic Supplementary Material M1–2). The harbour of Balatonfenyves in Hungary is a typical example for such a situation. Using drone polarimetry, recently Száz et al. (2023) measured the reflection–polarization characteristics of these dark lake patches from the viewpoint of flying polarotactic insects. The positive polarotaxis of chironomids suggests that such an artificial dark water patch may act as a supernormal stimulus. This may trigger a more intense in situ swarming and oviposition of chironomid imagoes due to the higher degree of polarization of water-reflected light. We hypothesized that the larger intensity of swarming at such a dark water patch and its richness in organic matter synergistically cause increased larval abundance in the bottom.
Contour of Lake Balaton, where the arrows mark the mouth of Zala River, Balatonmáriafürdő, and our study site, Balatonfenyves (a). Satellite photos of the mouth of Zala River (b photo by Google Earth; imagery date: 12 April 2016) and the harbour of Balatonfenyves (c photo by Google Earth; imagery date: 6 July 2017). Aerial photos of the harbour of Balatonfenyves and the surrounding lake (d, e photos by György Kriska and Ferenc Kriska). A satellite photo of the harbour of Balatonmáriafürdő (f photo by Google Earth; imagery date: 17 April 2010)
To test this hypothesis, we measured the reflection–polarization characteristics of the dark water of the harbour of Balatonfenyves and the neighbouring brighter lake water, followed by sampling larvae from the sediment to compare the amount of larvae inside and outside the harbour. We demonstrate here that spawning chironomids take advantage of polarized light reflected from the dark water rich in organic matter: we showed that polarotactic chironomids are intensely attracted to the strongly and horizontally polarizing seasonally dark water patch at the canal inflow, where they lay a vast amount of eggs, increasing the abundance of their larvae.
Materials and methods
Study site
Lake Balaton is the largest lake in Central Europe with a surface of 610 km2, whilst its average depth is only 3–4 m. The bed of the lake is asymmetric: the water gets deep more rapidly close to the shore at the northern bank, but the water remains shallow for several hundreds of metres at the southern bank. Since the investigated area was located at the southern bank, the water depth was only 1.5 m at distances of 300–500 m from the shore. Comparing the northern and southern littoral zones of the lake, the bottom compound is considerably different. At the northern bank, the hard bottom is covered by thick, watery mud, whilst at the southern bank, the bottom consists of sand. The asymmetric distribution is the result of the prevailing winds blowing from the north (Virág 1998).
The formation of waves is fostered by the shallowness of the lake and the relatively low density of the warm water which easily gets heated in summer. Currents evolve alongside the lake which is caused by local variations of the atmospheric pressure, aided by steady winds. The currents and the waves mix the water body easily, which results in a bright-coloured water. The high calcium content also contributes to the water brightness.
The Zala River is the main inflow of Balaton besides other small natural streams and manmade canals. The northern inflowing streams have typically karst waters originating from limestone or dolomite rocks. The water of the southern canals is dark and rich in humic substances, coming from marshes and agricultural inland waters. In some areas, the dark inflowing water can be seen in satellite photos as dark patches (Fig. 1b, c, f). Besides humic substances, these inflows carry considerable amount of nitrate and phosphate. These nutrients are the main reasons for the increased phytoplankton production in the south-west basin of the Lake Balaton (Virág 1998).
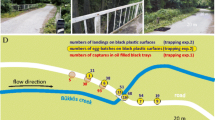
The harbour of Balatonfenyves (46° 42′ 54.693″ N, 17° 28′ 33.413″ E) has a water body separated from Lake Balaton with two parallel, 400-m-long concrete dykes (Fig. 1c–e). The water of the harbour mixes with the lake water only through a thin canal used by boats. The canal mouth is at the base of the harbour (Fig. 2), where water inflow is controlled with a water gate. From autumn to spring, the canal transports dark water with high concentration of dissolved humic substances. Between autumn and spring, the colour of harbour water turns almost black, whilst the surrounding lake water is significantly brighter khaki-green (Fig. 1c–e, Electronic Supplementary Material M1). The striking difference in the intensity of water-reflected light can also be seen on satellite images (Fig. 1c). After closing the water gate in May, due to the currents and boat traffic, the colour of the harbour water starts to lighten and from the end of July, no colour difference appears between the harbour and lake waters.
Sampling areas at Balatonfenyves. H: harbour, B1: Lake Balaton 30 m from the harbour, B2: Lake Balaton 200 m from the harbour. The white circular sectors represent the azimuthal field of view of our imaging-polarimetric measurement quintets, each consisting of five measurements. WN: western side, northern direction (Fig. 4), WS: western side, southern direction (Fig. 5), EN: eastern side, northern direction (Fig. 6), ES: eastern side, southern direction (Fig. 7). The solar elevation angle was 53°, and the azimuthal direction of the Sun is marked with a yellow arrow (photo by Google Earth; imagery date: 16 April 2016)
Imaging polarimetry
The reflection–polarization characteristics of the surface of harbour and lake water (Fig. 2) were measured with imaging polarimetry in the red (650 ± 50 nm), green (550 ± 50 nm) and blue (450 ± 50 nm) parts of the spectrum as described by Horváth and Varjú (1997). Measurements were performed at 28 May 2017 under clear sky conditions around noon at two (eastern, western) locations of the dyke for two (northern, southern) opposite directions of view as shown in Fig. 2. At the eastern and western side of the harbour, a pair of five imaging-polarimetric measurements were done: five towards north, and other five towards south, symmetrically to the harbour edge with the same camera settings of the polarimeter. Hence, four quintets of imaging-polarimetric measurements were made, and each quintet contained polarization patterns of the dark-watered harbour and the bright-watered lake. The optical axis of the polarimeter enclosed -37° with the horizontal, which is the Brewster’s angle relative to the horizon, from which the originally unpolarized light is reflected with a maximal degree of horizontal polarization. As a result, at each measurement site (marked with EN, ES, WN and WS in Fig. 2), we obtained the reflection–polarization characteristics of the dark harbour water and the brighter lake water. For each of the four sites, the reflection–polarization properties of the harbour- and lake-water-reflected light were compared by (i) defining masks for the harbour and lake water on the polarization patterns, and (ii) using a Monte Carlo approach described in the Statistics section.
Larval sampling
We performed our larval survey at the harbour of Balatonfenyves on 21 July 2017. This date was chosen by taking into account the swarming dynamics of the chironomid species living in Lake Balaton. In the lake’s bed, three chironomid species (Chironomus balatonicus [Devai, Wulker et Scholl, 1983], Procladius choreus [Meigen, 1804], Tanypus punctipennis [Meigen, 1818]) dominate. Based on the study of Specziár and Bíró (2000), we consider it as valid to our experimental site, as well. P. choreus and T. punctipennis species appear with at least two generations each year (Specziár 2008; Árva et al. 2015a). First, the overwintered larvae emerge. Typical periods of these events are May–June and February–June for P. choreus and T. punctipennis, respectively, whilst the second generation of the imagoes appears from mid-August to the end of September (Specziár and Vörös 2001; Specziár 2008). C. balatonicus develop only one generation per year, the overwintered larvae emerge from April to the beginning of July (Specziár 2008; Árva et al. 2015b). Since the shade of the harbour water is the darkest from the second part of autumn to the middle of spring, sampling in July enabled us to collect the most larvae, the parents of which swarmed when the water in the harbour was the darkest.
As shown in Fig. 2, we designed three sampling areas: one inside the harbour (H), one in Balaton, 30 m from the harbour to the east (B1) and one also in the lake, 200 m from the harbour towards east (B2). The average water depth in and outside the harbour was 2.5 m and 1–2 m, respectively. We took 15 sediment samples with a systematic, non-contiguous, qualitative sampling method from each of the three sampling areas to estimate the occurrence density of chironomid larvae. All samples were taken with a Ponar grab, the sampling area was 25 cm × 25 cm. The samples were washed through a net with a mesh size of 950 µm, and each sample was conserved in 75% ethanol. Finally, the chironomid larvae were selected and counted under a stereomicroscope later in the laboratory.
Estimation of larval biomass and average larval size
After selecting the chironomid larvae, each of the 45 collected samples were photographed in a Petri dish (diameter = 10 cm) from above (Fig. 3a). On each photo, every larva was manually masked in the red channel (red pixel intensity value was set to 255), and finally the layer of the masks was placed on a black background. In the case of overlapping larvae, the green or blue channels of the image were used for masking, so that each larva was represented by a bright blob (pixel intensity = 255) in one of the red, green, blue channels of the mask image (Fig. 3b). The number of these blobs gave the number of larvae for the current sample/photograph. The size of each larva was estimated by calculating the corresponding blob’s area in pixels and normalizing it with the area of the Petri dish measured also in pixels. Average size of larvae for a given photo was also calculated. Finally, to estimate the larval biomass for a given sample/photograph, the cumulative larval size was calculated.
A sample of chironomid larvae from sampling area H. a 8-bit RGB photograph of the larvae in the Petri dish. b The corresponding, manually created mask image for further evaluation. On the black background, larvae are primarily represented by red blobs by setting the pixel values to 255 in the red channel. In case of overlapping larvae, the same procedure was followed in the green or blue channels. The area of each coloured blob represents the size of a given larva
Organic matter content
Parallel with the larval samplings, on 21 July 2017, 50 mL portions of the sediment samples were separated for measuring the organic matter content as follows: the sample weight was measured after drying at 105 °C, then after being kept at 600 °C for 2 h, the mass difference between the dried and incinerated weight resulted in the organic matter content.
Statistics
The number of masked pixels which were taken into account for characterizing the degree and angle of polarization of water-reflected light on the lake and harbour side was around 1 million per side. Therefore, performing a statistical test, for example, for comparing the degree of polarization on the harbour and lake side at a given measurement site would result in extremely high significance due to the high sample size (Lin et al. 2013). Thus, we applied the method of Pereszlényi et al. (2021). We randomly selected 100 degree of polarization values from the harbour, as well as from the lake side for a given measurement location and compared the samples with Mann–Whitney U test. We repeated this procedure 10,000 times and averaged the statistical results. The same was applied for the angle of polarization measurements.
We used Kruskal–Wallis tests followed by pairwise Wilcoxon rank sum tests with Bonferroni’s correction to reveal differences in larval abundance, larval size, larval biomass and organic matter content across the sampling areas. For these variables, we also applied principal component analysis (PCA) and fitted ellipses to component scores with 95% confidence interval. The statistical tests were performed with the R-3.6.3 statistical package (R Core Team 2020).
Ethics
The studied insects are non-protected in Hungary; thus, no approval from any ethical committee was necessary for our work.
Results
Reflection polarization of water surfaces
Figure 4 shows the five measured reflection–polarization patterns of the eastern side of the dark-watered harbour in the blue spectral range, when the polarimeter faced north (circle segment EN in Fig. 2). The polarization patterns of the other three measurements (each consisting of five imaging-polarimetric measurements) at the eastern side facing south (ES), western side facing north (WN) and western side facing south (WS) are displayed in Figs. 5, 6 and 7, respectively.
Reflection–polarization patterns of the surface at the dark harbour water and the bright lake water measured at the eastern side of the harbour with north-facing polarimeter (EN in Fig. 2) in the blue (450 nm) part of the spectrum. Five measurements were performed symmetrically to the edge of the harbour. a Colour photographs. b Patterns of the degree of linear polarization d. c Patterns of the angle of polarization α measured clockwise from the vertical. Dashed lines represent the regions/masks where d and α values were compared for the harbour and lake side
Boxplots in Fig. 8a show the measured degree of polarization of light reflected from the harbour and lake water in the blue spectral range for all measurement sites. For the quintets of imaging-polarimetric measurements marked with EN, ES, WN and WS in Fig. 2, the median of the degree of polarization of light reflected from the dark harbour water was 1.88, 1.63, 1.31 and 1.23 times higher than that of the brighter lake-water-reflected light, respectively. Results of statistical comparisons between the degree of polarization of water-reflected light from the harbour and lake are shown in Supplementary Table S1. For all four measurement sites, highly significant differences were obtained. Since polarization sensitivity of aquatic insects operates typically in the UV or blue spectral region (Schwind 1995), we did not plot the results for the green and red channels of the polarimeter, but those results were qualitatively similar.
Boxplots of degree d (a) and angle α (b) (measured clockwise from the vertical) of linear polarization values within the masked areas for all four imaging-polarimetric measurement quintets EN, ES, WN and WS (Figs. 2, 4, 5, 6 and 7) in the blue spectral range. Outliers are not shown. Grey and white boxes show d and α corresponding to the dark harbour water and the brighter lake water, respectively. The horizontal dashed line in B at α = 90° shows the horizontally polarized direction
According to Fig. 8b, the direction (angle) of polarization of water-reflected light was not exactly horizontal, but varied around the horizontal direction, which is indicated by a horizontal dashed line in the graph. Since the deviations from horizontal depended on the position of the Sun relative to the polarimeter, the reason for the slight differences in the direction of polarization from the horizontal was the presence of direct sunlight.
Abundance of chironomid larvae
Boxplots in Fig. 9 show the results of larval samplings. Figure 9a displays the numbers of chironomid larvae collected at sampling areas H, B1 and B2, where the mean number of caught individuals (black diamonds) are 93, 18.7 and 7.5 specimens, respectively. The Kruskal–Wallis test indicated significant differences between the sampling areas (χ2 = 28.06, df = 2, p < 0.0001). According to multiple comparisons, there were significantly more chironomid larvae in the seasonally dark harbour (H) than in the surrounding lake (H vs. B1: p = 0.00014; H vs. B2: p < 0.0001). Between sampling areas B1 and B2, the difference was not significant (p = 0.119).
Boxplots of the output data for the 15 larval samplings at each of the three sampling areas H, B1 and B2. a Number of chironomid larvae, b larval biomass, c average larval size and d organic matter content. Black diamonds show average values, and significances obtained from the pairwise Wilcoxon rank sum tests are displayed at the top of each subfigure (NS: not significant, *: p < 0.05, **: p < 0.01, ***: p < 0.001)
Biomass and average size of chironomid larvae
As shown in Fig. 9b, the biomass of chironomid larvae was 10.34 times higher in the seasonally dark harbour water (H) than in the seasonally bright lake water next to the harbour (B1). The larval biomass was 2.97 times higher in the sampling area B1 than in B2. Here, the larval biomass values are in arbitrary units, describing the pixel coverage percentages in the Petri dish as described in the Methods section. For example, the value of 0.1 means that 10% of the Petri dish’s area was covered by larvae (overlaps between larvae were taken into account). The statistical analysis showed significant differences between the sampling areas (χ2 = 32.16, df = 2, p < 0.0001). The results of all pairwise comparisons were significant (H vs. B1 and H vs. B2: p < 0.0001; B1 vs. B2: p < 0.014).
Similar to the larval biomass results, the average larval size was also highest in the harbour: 2.05 times larger than in the sampling area B1, whilst it was 1.69 times larger in the sampling area B1 than in B2 (Fig. 9c). Arbitrary units of the average larval size are the same as described for Fig. 9b. Kruskal–Wallis test showed significant differences between the sampling areas (χ2 = 19.60, df = 2, p < 0.0001). The average larval size in the harbour was significantly higher than in the two outer sampling areas B1 and B2 (H vs. B1: p = 0.0186; H vs. B2: p = 0.00019), whilst it was not statistically different between B1 and B2 (B1 vs. B2: p = 0.054).
Organic matter content
The organic matter contents of the sampling areas are shown in Fig. 9d. In both sampling areas B1 and B2 of the lake, we found one outlier data which was not used in the statistical analysis. Kruskal–Wallis test showed significant differences between the three sampling areas (χ2 = 17.49, df = 2, p < 0.0005). The organic matter content of the harbour was about 2 times greater and significantly higher than that of sampling areas B1 and B2, according to the pairwise comparisons (H vs. B1: p < 0.001; H vs. B2: p = 0.0046). There was no significant difference (p = 0.605) between the B1 and B2 sampling areas.
Principal component analysis
The principal component analysis showed (Fig. 10) that the scatter (characterized by confidence ellipses) of samples B1 and B2 highly overlap. There are only three samples from B1 sampling area outside of the B2 confidence ellipse. In contrast to the lake samples, the scatter of the harbour samples covers a much bigger area. The average size, abundance and biomass of larvae as well as the organic matter content in the bottom of the lake are more homogeneous than in the harbour. The average larval size is independent of the larval abundance. This suggests that in the harbour, not the food source is the limiting factor for chiromonid larvae. The larval biomass strongly and positively correlates with the organic matter content. The larval biomass and organic content positively correlate with both the larval abundance and average size; however, this correlation is not so strong. The first two principal components explained 93.1% of the total variance (Table 1).
Principal component analysis plot of the first two principal components PC1 and PC2 of the variables larval abundance, larval biomass, average larval size and organic matter content. Ellipses are fitted to the scores with 95% confidence interval. Table 1 contains the total variance explained by each component
Discussion
The water of Zala River and the southern canals is dark and rich in humic substances. Due to currents, a dark inflowing water can only be seen when the river’s discharge is big enough (like the Zala River in Fig. 1b, Electronic Supplementary Material M3), or the dispersion is blocked with dykes (like at the harbours of Balatonfenyves and Balatonmáriafürdő in Fig. 1c–f and Electronic Supplementary Material M1–2). The changes in the shade of water colour go hand in hand with variations of the polarization characteristics of water-reflected light. In the case of bright waters, besides surface reflection, light is also returned by the suspended materials (sand or calcium carbonate) and the bottom if the water is shallow enough. This returned light becomes partially vertically polarized after refraction at the water–air interface. The vertically polarized component is mixed with the horizontally polarized light reflected from the water surface, which results in almost unpolarized light, if the intensities of both components are nearly equal. The dissolved humic substances in dark waters absorb light; thus, the vertically polarized component is much weaker. Therefore, dark water patches, like the harbour water in our experimental site, reflect horizontally polarized light with higher degrees of polarization than the bright lake water (Figs. 4, 5, 6, 7 and 8). At Balatonfenyves, a small canal flows into the lake, which carries dark-brown water being rich in humic substances. Before the dykes had been built in 2015, dark water patches could not be seen at the mouth of the canal, because the lake currents dispersed the dark inflowing water quickly.
Counting the egg-laying females or the spawn clumps (which directly denote the attractiveness of water bodies of different shades) was not possible. It is unknown whether the predation rate and other factors were identical at the experimental sites. We counted significantly more larvae in the seasonally dark harbour water than in the brighter lake water. There was also significant difference in larval biomass between the two sampling areas in the lake: at site B1 (next to the harbour), the density of chironomid larvae was significantly higher than at B2. Since we have measured the highest organic matter contents at the harbour (H), but have not found significant difference between sites B1 and B2, the increased larval abundance of site B1 compared to B2 cannot be explained by the changes of organic matter content. Thus, our results suggest that the harbour’s dark, and thus highly and nearly horizontally polarizing surface attracted female chironomid imagoes by positive polarotaxis.
Besides the increased larval density of chironomids, the average larval size was also significantly higher at the harbour. The increased inflow of nutrients from the canal increases the organic compound concentration in the mud which provides ideal circumstances for chironomid larvae to grow. According to Hooper et al. (2003), the higher the organic compound concentration in the mud, the higher is the growth and survival rate of chironomid larvae. This is also supported by our measurements of the larval biomass and organic matter content. We found significantly higher larval biomass and average larval size in the sediment of the seasonally dark harbour compared to sites B1 and B2 in the brighter lake water.
The attractiveness of a water surface reflecting horizontally polarized light to aquatic insects depends on the illumination, water depth, water brightness determined by the concentration of floating materials, the observation angle and the polarization sensitivity threshold of a given aquatic insect species (Kriska et al. 2009). Our results confirm that the water brightness plays an important role in habitat selection of chironomids. The physical, chemical and biological parameters like water turbidity, abundance of aquatic plants, predation pressure are unknown for flying female imagoes when searching for a sufficient place for egg laying. However, the degree of horizontal polarization of water-reflected light can be remotely sensed and is a reliable physical signal of a sufficient spawning area (Bernáth et al. 2002).
In our study area (throughout the southern bank of Lake Balaton likewise), the bottom is composed of sand with low concentration of particulate organic matter. However, where light reaches the bottom of shallow waters, algae biofilm can develop resulting in higher nutrient concentration of the sediment. Our results suggest that the dark and thus more polarizing water accumulated in the studied harbour attracts female chironomids to a better-quality swarming area, which is a beneficial effect for the population. In many cases, manmade dark surfaces reflecting highly and horizontally polarized light have negative impacts on polarotactic aquatic insects. This phenomenon is the so-called polarized light pollution (Horváth et al. 2009). In our study we have described a situation when the presence of an artificially evolved highly and horizontally polarizing dark water patch has advantageous consequences (increased abundance and biomass of larvae) for chironomids. During our samplings, we concentrated on chironomids, because at Lake Balaton, this is the most abundant group of aquatic insects that was previously shown to be positively polarotactic (Lerner et al. 2008, 2011; Meltser et al. 2008; Horváth et al. 2011; Robertson et al. 2018). However, imaging polarimetry revealed that the strongly polarizing dark water in the harbour is most likely more attractive for polarotactic aquatic insects than the surrounding weakly polarizing lake water. It should be examined, how other aquatic insects are affected by the optical signal of such a dark-watered harbour. Our results also support suggestions that the polarization characteristics of water-reflected light can be an optical indicator of water quality for chironomid larval development (Bernáth et al. 2002; Lerner et al. 2008, 2011; Meltser et al. 2008). On the other hand, it is well known that the mass swarming of non-biting midges in urbanized areas often causes considerable nuisance for humans (Ali 1980; Lin and Quek 2011); thus, such dark water patches near the shore of lakes may amplify mass swarmings close to urbanized areas. Future research has to examine whether a dark-watered harbour can indeed locally enhance remarkably the swarming intensity. If the answer is yes, it would be worth making efforts to artificially mix the dark harbour water with the surrounding bright lake water.
The measured change in the polarization patterns of the studied lake surface with inflowing dark water and the resulting increase of chironomid larval abundance and biomass may also occur at several similar sites. Since this phenomenon can also occur in other lakes, researchers should take into consideration reflection–polarization characteristics when studying and explaining the larval abundance of aquatic insects.
Data availability statement
All relevant data are within the paper.
References
Ali A (1980) Nuisance chironomids and their control: a review. Bull Entomol Soc Am 26:3–16
Armitage PD (1995) Behaviour and ecology of adults. In: Armitage P, Cranston PS, Pinder LCV (eds) The chironomidae. The biology and ecology of non-biting midges. Chapter 9. Chapman & Hall, London - New York - Tokyo – Melbourne, pp 194–224
Árva D, Specziár A, Erős T, Tóth M (2015a) Effects of habitat types and within lake environmental gradients on the diversity of chironomid assemblages. Limnologica 53:26–34
Árva D, Tóth M, Horváth H, Nagy SA, Specziár A (2015b) The relative importance of spatial and environmental processes in distribution of benthic chironomid larvae within a large and shallow lake. Hydrobiologia 742:249–266
Bernáth B, Szedenics G, Wildermuth H, Horváth G (2002) How can dragonflies discern bright and dark waters from a distance? The degree of polarisation of reflected light as a possible cue for dragonfly habitat selection. Freshw Biol 47:1707–1719
Cranston PS (1995) Introduction. In: Armitage P, Cranston PS, Pinder LCV (eds) The chironomidae. The biology and ecology of non-biting midges. Chapman & Hall, London - New York - Tokyo – Melbourne, pp.. 1–7
Egri Á, Pereszlényi Á, Farkas A, Horváth G, Penksza K, Kriska G (2017) How can asphalt roads extend the range of in situ polarized light pollution? A complex ecological trap of Ephemera danica and a possible remedy. J Insect Behav 30:374–384
Hooper HL, Sibly RM, Hutchinson TH, Maund SJ (2003) The influence of larval density, food availability and habitat longevity on the life history and population growth rate of the midge Chironomus riparius. Oikos 102:515–524
Horváth G (1995) Reflection-polarization patterns at flat water surfaces and their relevance for insect polarization vision. J Theor Biol 175:27–37
Horváth G (ed) (2014) Polarized light and polarization vision in animal sciences. Springer, Heidelberg, Berlin, London, New York
Horváth G, Kriska G (2008) Polarization vision in aquatic insects and ecological traps for polarotactic insects. In: Lancaster J, Briers RA (eds) Aquatic insects: challenges to populations. CAB International Publishing, Wallingford, pp 204–229
Horváth G, Varjú D (1997) Polarization pattern of freshwater habitats recorded by video polarimetry in red, green and blue spectral ranges and its relevance for water detection by aquatic insects. J Exp Biol 200:1155–1163
Horváth G, Varjú D (2004) Polarized light in animal vision—polarization patterns in nature. Springer, Heidelberg, Berlin, New York
Horváth G, Zeil J (1996) Kuwait oil lakes as insect traps. Nature 379:303–304
Horváth G, Kriska G, Malik P, Robertson B (2009) Polarized light pollution: a new kind of ecological photopollution. Front Ecol Environ 7:317–325. https://doi.org/10.1890/080129
Horváth G, Móra A, Bernáth B, Kriska G (2011) Polarotaxis in non-biting midges: female chironomids are attracted to horizontally polarized light. Physiol Behav 104:1010–1015
Kriska G, Horváth G, Andrikovics S (1998) Why do mayflies lay their eggs en masse on dry asphalt roads? Water-imitating polarized light reflected from asphalt attracts Ephemeroptera. J Exp Biol 201:2273–2286
Kriska G, Malik P, Szivák I, Horváth G (2008) Glass buildings on river banks as “polarized light traps” for mass-swarming polarotactic caddis flies. Naturwissenschaften 95:461–467
Kriska G, Bernáth B, Farkas R, Horváth G (2009) Degrees of polarization of reflected light eliciting polarotaxis in dragonflies (Odonata), mayflies (Ephemeroptera) and tabanid flies (Tabanidae). J Insect Physiol 55:1167–1173
Lerner A, Meltser N, Sapir N, Erlick C, Shashar N, Broza M (2008) Reflected polarization guides chironomid females to oviposition sites. J Exp Biol 211:3536–3543
Lerner A, Sapir N, Erlick C, Meltser N, Broza M, Shashar N (2011) Habitat availability mediates chironomid density-dependent oviposition. Oecologia 165:905–914
Lin YJ, Quek RF (2011) Observations on mass emergence of chironomids (Diptera: Chironomidae) in Bedok, Singapore with notes on human-chironomid interactions. Nat Singap 4:339–347
Lin M, Lucas HC, Shmueli G (2013) Too big to fail: Large samples and the p-value problem. Inf Syst Res 24:906–917
Meltser N, Kashi Y, Broza M (2008) Does polarized light guide chironomids to navigate toward water surfaces? Boletim Do Museu Municipal Do Funchal (história Natural) 13:141–149
Pereszlényi Á, Száz D, Jánosi IM, Horváth G (2021) A new argument against cooling by convective air eddies formed above sunlit zebra stripes. Sci Rep 11:15797
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Robertson BA, Keddy-Hector IA, Shrestha SD, Silverberg LY, Woolner CE, Hetterich I, Horváth G (2018) Susceptibility to ecological traps is similar among closely related taxa but sensitive to spatial isolation. Anim Behav 135:77–84
Schwind R (1991) Polarization vision in water insects and insects living on a moist substrate. J Comp Physiol A 169:531–540
Schwind R (1995) Spectral regions in which aquatic insects see reflected polarized light. J Comp Physiol A 177:439–448
Specziár A (2008) Life history patterns of Procladius choreus, Tanypus punctipennis and Chironomus balatonicus in Lake Balaton. Int J Limnol 44:181–188
Specziár A, Bíró P (2000) Spatial distribution and short-term changes of the benthic chironomid fauna in Lake Balaton during 1995 and 1998. Állattani Közlemények 85:93–107 ((in Hungarian))
Specziár A, Vörös L (2001) Long term dynamics of Lake Balaton’s chironomid fauna and its dependence on the phytoplankton production. Arch Hydrobiol 152:119–142
Száz D, Takács P, Bernáth B, Kriska G, Barta A, Pomozi I, Horváth G (2023) Drone-based imaging polarimetry of dark lake patches from the viewpoint of flying polarotactic insects with ecological implication. Remote Sens 15(11):2797. https://doi.org/10.3390/rs15112797
Virág Á (1998) The History and Present of Lake Balaton. Eger, Egri Nyomda, p 904 (in Hungarian)
Acknowledgements
Project no. 131738 has been implemented with the support to Ádám Egri provided from the National Research, Development and Innovation Fund of Hungary, financed under the PD_19 funding scheme. The project was also supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences obtained by Ádám Egri. Dénes Száz was supported by the Hungarian UNKP-21-4 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund. We are grateful to Gábor Emődi and Varga Bálint, the Facility Mangers of the Fenyves Yacht Club who permitted our field measurements in Balatonfenyves. We are also grateful to Béla Csányi for his help in the samplings, Gyula Záray and Péter Dobosy for their help in the organic compound concentration measurements.
Funding
Open access funding provided by ELKH Centre for Ecological Research.
Author information
Authors and Affiliations
Contributions
All authors gave final approval for publication and agree to be held accountable for the work performed therein. Substantial contributions to conception and design, drafting the article and revising it critically: ÁE, ÁP, JS, DS, GH, GK. Software development: ÁE, ÁP. Statistical analysis: ÁE, ÁP, DS. Data visualization, data analysis and interpretation: ÁE, ÁP, JS, DS, GH, GK.
Corresponding author
Ethics declarations
Conflict of interest
We declare we have no competing interests.
Ethical statement
For our study, no permission, licence or approval was necessary.
Additional information
Handling Editor: Noboru Okuda.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary File2 (MP4 32817 KB)
Supplementary file3 (MP4 16167 KB)
Supplementary file4 (MP4 38401 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Egri, Á., Pereszlényi, Á., Szekeres, J. et al. Ecological advantage of polarized light pollution: positive effect of a dark lake patch at a canal inflow on habitat of non-biting midges. Limnology 25, 97–109 (2024). https://doi.org/10.1007/s10201-023-00733-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-023-00733-6