Abstract
Objective
To review and assess the quality of the available evidence on the cost-effectiveness of erlotinib in the first-line treatment of advanced non-small cell lung cancer (NSCLC).
Methods
A systematic review was conducted to identify full-text original economic evaluations of erlotinib in the first-line treatment of advanced NSCLC written in English and published from the year 2000 onwards. Study characteristics and results were recorded and compared. The quality of the studies was assessed by the Quality of Health Economic Studies (QHES) questionnaire.
Results
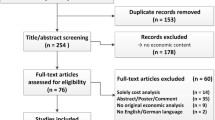
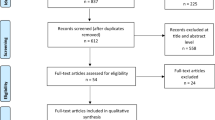
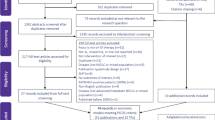
Eleven out of 130 papers were chosen for this review. Comparative regimens consisted of a best supportive care, reverse strategy, bevacizumab, cisplatin plus pemetrexed, carboplatin plus gemcitabine or gefitinib. The methods most used in these studies were modeling and sensitivity analysis and cost-effectiveness analysis. All of the studies evaluated direct costs and used quality-adjusted life-year (QALY) and life-years gained (LYG) as outcome, with 3% and 3.5% discount rate. The studies assigned ICER that ranged from dominant to I$305,510.31/QALY and from I$31,209.55/LYG to I$66,540.20/LYG. Based on the willingness to pay threshold, seven studies concluded that erlotinib was cost-effective, two studies showed that erlotinib was cost-effective on specific patients with certain conditions, and two studies comparing erlotinib with reverse strategy did not find a difference in cost-effectiveness. The high quality of these studies was confirmed using the QHES tool: the mean score was 75.77 out of 100 (SD 9.38).
Conclusion
Most of these high-quality studies suggested that erlotinib was cost-effective in the first-line treatment of advanced NSCLC.




Similar content being viewed by others
References
Siracusa, M., Grappasonni, I., Petrelli, F.: The pharmaceutical care and the rejected constitutional reform: what might have been and what is. Acta Biomed. 88(3), 352–359 (2017). https://doi.org/10.23750/abm.v88i3.6376
Signorelli, C., Odone, A., Gozzini, A., Petrelli, F., Tirani, M., Zangrandi, A., Zoni, R., Florindo, N.: The missed constitutional reform and its possible impact on the sustainability of the Italian National Health Service. Acta Biomed. 88(1), 91–94 (2017). https://doi.org/10.23750/abm.v88i1.6408
International Agency for Research on Cancer, World Health Organization. Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. Press Release no. 263. https://www.who.int/cancer/PRGlobocanFinal.pdf. Accessed 12 Sept 2018
Petrelli, F., Scuri, S., Tanzi, E., Nguyễn, T.T.C., Grappasonni, I.: Lifestyles and discomfort in a sample of young Romanian students. J Prev Med Hyg. 59(3), E230–E235 (2018). https://doi.org/10.15167/2421-4248/jpmh2018.59.3.985
Grappasonni, I., Scuri, S., Tanzi, E., Kracmarova, L., Petrelli, F.: The economic crisis and lifestyle changes: a survey on frequency of use of medications and of preventive and specialistic medical care, in the Marche Region (Italy). Acta Biomed. 89(1), 87–92 (2018). https://doi.org/10.23750/abm.v89i1.7068
Siracusa, M., Petrelli, F.: Trade of food supplement: food or drug supplement?. Recenti Prog Med. 107(9), 465–471 (2016). https://doi.org/10.1701/2354.25224
Grappasonni, I., Marconi, D., Mazzucchi, F., Petrelli, F., Scuri, S., Amenta, F.: Survey on food hygiene knowledge on board ships. Int Marit Health 64(3), 160–167 (2013)
Grappasonni, I., Petrelli, F., Klusoňová, H., Kračmarová, L.: Level of understanding of medical terms among italian students. Ceska Slov Farm. Winter 65(6), 216–220 (2016)
Spacilova, L., Klusonova, H., Petrelli, F., Signorelli, C., Visnovsky, P., Grappasonni, I.: Substance use and knowledge among Italian high school students. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 153(2), 163–168 (2009)
Kračmarová, L., Klusoňová, H., Petrelli, F., Grappasonni, I.: Tobacco, alcohol and illegal substances: experiences and attitudes among Italian university students. Rev. Assoc. Med. Bras. (1992) 57(5), 523–528 (2011)
Petrelli, F., Grappasonni, I., Peroni, A., Kracmarova, L., Scuri, S.: Survey about the potential effects of economic downturn on alcohol consumption, smoking and quality of life in a sample of Central Italy population. Acta Biomed. 89(1), 93–98 (2018). https://doi.org/10.23750/abm.v89i1.7059
Grappasonni, I., Petrelli, F., Amenta, F.: Deaths on board ships assisted by the Centro Internazionale Radio Medico in the last 25 years. Travel Med Infect Dis. 10(4), 186–191 (2012). https://doi.org/10.1016/j.tmaid.2012.06.006
Ferlay, J.S.-F., Lortet-Tieulent, E., Rosso, J., Coebergh, S., Jan-Willem, W., Comber, H., Forman, D., Bray, F.: Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6), 1374–1403 (2013)
Ferlay, J., Ervik, I.S.,M., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D.M., Forman, D., Bray, F.: Cancer Incidence and Mortality Worldwide. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (2012). Accessed 15 Dec 2016
Siegel, R., Naishadham, D., Jemal, A.: Cancer statistics, 2013. CA Cancer J. Clin. 63(1), 11–30 (2013)
Navada, S., Lai, P., Schwartz, A., Kalemkerian, G.: Temporal trends in small cell lung cancer: analysis of the national Surveillance, Epidemiology, and End-Results (SEER) database. J. Clin. Oncol. 24(18_suppl), 7082–7082 (2006)
Sher, T., Dy, G.K., Adjei, A.A.: Small cell lung cancer. In: Mayo Clinic Proceedings 2008, vol. 3, pp. 355–367. Elsevier
Surveillance, E., and End Results Program: SEER stat fact sheets: lung and bronchus cancer. http://seer.cancer.gov/statfacts/html/lungb.html (2013). Accessed 15 Oct 2017
Spiro, S.G., Gould, M.K., Colice, G.L.: Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes: ACCP evidenced-based clinical practice guidelines (2nd edn.). Chest 132(3 Suppl), 149 s–160 s (2007). https://doi.org/10.1378/chest.07-1358
Cuccioloni, M., Bonfili, L., Mozzicafreddo, M., Cecarini, V., Scuri, S., Cocchioni, M., Nabissi, M., Santoni, G., Eleuteri, A.M., Angeletti, M.: Mangiferin blocks proliferation and induces apoptosis of breast cancer cells via suppression of the mevalonate pathway and by proteasome inhibition. Food Funct. 7(10), 4299–4309 (2016)
Shi, Y., Au, J.S., Thongprasert, S., Srinivasan, S., Tsai, C.M., Khoa, M.T., Heeroma, K., Itoh, Y., Cornelio, G., Yang, P.C.: A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. 9(2), 154–162 (2014). https://doi.org/10.1097/jto.0000000000000033
Marchetti, A., Martella, C., Felicioni, L., Barassi, F., Salvatore, S., Chella, A., Camplese, P.P., Iarussi, T., Mucilli, F., Mezzetti, A., Cuccurullo, F., Sacco, R., Buttitta, F.: EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J. Clin. Oncol. 23(4), 857–865 (2005). https://doi.org/10.1200/jco.2005.08.043
Doval, D., Prabhash, K., Patil, S., Chaturvedi, H., Goswami, C., Vaid, A., Desai, S., Dutt, S., Veldore, V., Jambhekar, N., Mehta, A., Hazarika, D., Azam, S., Gawande, S., Gupta, S.: Clinical and epidemiological study of EGFR mutations and EML4-ALK fusion genes among Indian patients with adenocarcinoma of the lung. Oncotargets Ther. 8, 117–123 (2015). https://doi.org/10.2147/ott.s74820
Sharma, S.V., Bell, D.W., Settleman, J., Haber, D.A.: Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 7(3), 169–181 (2007). https://doi.org/10.1038/nrc2088
Cioffi, P., Marotta, V., Fanizza, C., Giglioni, A., Natoli, C., Petrelli, F., Grappasonni, I.: Effectiveness and response predictive factors of erlotinib in a non-small cell lung cancer unselected European population previously treated: a retrospective, observational, multicentric study. J. Oncol. Pharm. Pract. 19(3), 246–253 (2013). https://doi.org/10.1177/1078155212465994
De Grève, J., Van Meerbeeck, J., Vansteenkiste, J.F., Decoster, L., Meert, A.P., Vuylsteke, P., Focan, C., Canon, J.L., Humblet, Y., Berchem, G., Colinet, B., Galdermans, D., Bosquée, L., Vermeij, J., Dewaele, A., Geers, C., Schallier, D., Teugels, E.: Prospective evaluation of first-line erlotinib in advanced non-small cell lung cancer (NSCLC) carrying an activating egfr mutation: a multicenter academic phase II study in caucasian patients (FIELT). PLoS One (2016). https://doi.org/10.1371/journal.pone.0147599
Zhou, C., Wu, Y.L., Chen, G., Feng, J., Liu, X.Q., Wang, C., Zhang, S., Wang, J., Zhou, S., Ren, S., Lu, S., Zhang, L., Hu, C., Hu, C., Luo, Y., Chen, L., Ye, M., Huang, J., Zhi, X., Zhang, Y., Xiu, Q., Ma, J., Zhang, L., You, C.: Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 12(8), 735–742 (2011). https://doi.org/10.1016/s1470-2045(11)70184-x
Rosell, R., Carcereny, E., Gervais, R., Vergnenegre, A., Massuti, B., Felip, E., Palmero, R., Garcia-Gomez, R., Pallares, C., Sanchez, J.M., Porta, R., Cobo, M., Garrido, P., Longo, F., Moran, T., Insa, A., De Marinis, F., Corre, R., Bover, I., Illiano, A., Dansin, E., de Castro, J., Milella, M., Reguart, N., Altavilla, G., Jimenez, U., Provencio, M., Moreno, M.A., Terrasa, J., Munoz-Langa, J., Valdivia, J., Isla, D., Domine, M., Molinier, O., Mazieres, J., Baize, N., Garcia-Campelo, R., Robinet, G., Rodriguez-Abreu, D., Lopez-Vivanco, G., Gebbia, V., Ferrera-Delgado, L., Bombaron, P., Bernabe, R., Bearz, A., Artal, A., Cortesi, E., Rolfo, C., Sanchez-Ronco, M., Drozdowskyj, A., Queralt, C., de Aguirre, I., Ramirez, J.L., Sanchez, J.J., Molina, M.A., Taron, M., Paz-Ares, L.: Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13(3), 239–246 (2012). https://doi.org/10.1016/s1470-2045(11)70393-x
Cioffi, P., Laudadio, L., Nuzzo, A., Belfiglio, M., Petrelli, F., Grappasonni, I.: Gemcitabine-induced posterior reversible encephalopathy syndrome: a case report. J. Oncol. Pharm. Pract. 18(2), 299–302 (2012). https://doi.org/10.1177/1078155211424628
World Bank: World development indicators: consumer price index. http://databank.worldbank.org/data/reports.aspx?source=2&series=FP.CPI.TOTL&country=(2015). Accessed 17 July 2015
Ofman, J.J., Sullivan, S.D., Neumann, P.J., Chiou, C.F., Henning, J.M., Wade, S.W., Hay, J.W.: Examining the value and quality of health economic analyses: implications of utilizing the QHES. J. Manag. Care Pharm. JMCP. 9(1), 53–61 (2003). https://doi.org/10.18553/jmcp.2003.9.1.53
Vergnenegre, A., Massuti, B., de Marinis, F., Carcereny, E., Felip, E., Do, P., Sanchez, J.M., Paz-Arez, L., Chouaid, C., Rosell, R.: Economic analysis of first-line treatment with erlotinib in an EGFR-mutated population with advanced NSCLC. J. Thorac. Oncol. 11(6), 801–807 (2016)
Khan, I., Morris, S., Hackshaw, A., Lee, S.-M.: Cost-effectiveness of first-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy. BMJ Open 5(7), e006733 (2015)
Schremser, K., Rogowski, W.H., Adler-Reichel, S., Tufman, A.L., Huber, R.M., Stollenwerk, B.: Cost-effectiveness of an individualized first-line treatment strategy offering erlotinib based on EGFR mutation testing in advanced lung adenocarcinoma patients in Germany. Pharmacoeconomics 33(11), 1215–1228 (2015)
Chouaid, C., Crequit, P., Borget, I., Vergnenegre, A.: Economic evaluation of first-line and maintenance treatments for advanced non-small cell lung cancer: a systematic review. Clinicoecon. Outcomes Res. CEOR. 7, 9–15 (2015). https://doi.org/10.2147/ceor.s43328
Chouaid, C., Le Caer, H., Corre, R., Crequit, J., Locher, C., Falchero, L., Dujon, C., Berard, H., Monnet, I., Vergnenegre, A.: Cost analysis of erlotinib versus chemotherapy for first-line treatment of non-small-cell lung cancer in frail elderly patients participating in a prospective phase 2 study (GFPC 0505). Clin. Lung Cancer 14(2), 103–107 (2013)
Vergnenègre, A., Ray, J.A., Chouaid, C., Grossi, F., Bischoff, H.G., Heigener, D.F., Walzer, S.: Cross-market cost-effectiveness analysis of erlotinib as first-line maintenance treatment for patients with stable non-small cell lung cancer. Clinicoecon. Outcomes Res. CEOR 4, 31 (2012)
Walleser, S., Ray, J., Bischoff, H., Vergnenègre, A., Rosery, H., Chouaid, C., Heigener, D., de Castro Carpeño, J., Tiseo, M., Walzer, S.: Maintenance erlotinib in advanced nonsmall cell lung cancer: cost-effectiveness in EGFR wild-type across Europe. Clinicoecon. Outcomes Res. CEOR 4, 269 (2012)
Lee, V.W., Schwander, B., Lee, V.H.: Effectiveness and cost-effectiveness of erlotinib versus gefitinib in first-line treatment of epidermal growth factor receptor-activating mutation-positive non-small-cell lung cancer patients in Hong Kong. Hong Kong Med. J. 20(3), 178–186 (2014)
Wang, S., Peng, L., Li, J., Zeng, X., Ouyang, L., Tan, C., Lu, Q.: A trial-based cost-effectiveness analysis of erlotinib alone versus platinum-based doublet chemotherapy as first-line therapy for Eastern Asian nonsquamous non-small-cell lung cancer. PLoS One 8(3), e55917 (2013)
Lv, F., Yu, K., Gao, W., Yu, S.: Cost-utility of erlotinib combined with bevacizumab versus bevacizumab alone after completion of chemotherapy with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J. Chin. Pharm. Sci. 26(6), 447–454 (2017)
Ting, J., Ho, P.T., Xiang, P., Sugay, A., Abdel-Sattar, M., Wilson, L.: Cost-effectiveness and value of information of erlotinib, afatinib, and cisplatin-pemetrexed for first-line treatment of advanced EGFR mutation-positive non-small-cell lung cancer in the United States. Value Health 18(6), 774–782 (2015)
Christos, C., Chrystelle, L., Cecile, D., Pascal, T., Bernard, A.J., Isabelle, M., Alain, V.: Cost effectivenes of erlotinib versus chemotherapy for first-line treatment of non small cell lung cancer (NSCLC) in fit elderly patients participating in a prospective phase 2 study (GFPC 0504). BMC Cancer 12(1), 301 (2012)
Marshall, D.A., Donald, F., Lacny, S., Reid, K., Bryant-Lukosius, D., Carter, N., Charbonneau-Smith, R., Harbman, P., Kaasalainen, S., Kilpatrick, K.: Assessing the quality of economic evaluations of clinical nurse specialists and nurse practitioners: a systematic review of cost-effectiveness. NursingPlus Open 1, 11–17 (2015)
Jäkel, A., Plested, M., Dharamshi, K., Modha, R., Bridge, S., Johns, A.: A systematic review of economic evaluations in second and later lines of therapy for the treatment of non-small cell lung cancer. Appl. Health Econ. Health Policy 11(1), 27–43 (2013)
Lange, A., Prenzler, A., Frank, M., Golpon, H., Welte, T., von der Schulenburg, J.-M.: A systematic review of the cost-effectiveness of targeted therapies for metastatic non-small cell lung cancer (NSCLC). BMC Pulm. Med. 14(1), 192 (2014)
Lyseng-Williamson, K.A.: Erlotinib: a pharmacoeconomic review of its use in advanced non-small cell lung cancer. Pharmacoeconomics. 28(1), 75–92 (2010). https://doi.org/10.2165/10482880-000000000-00000
Bongers, M.L., Coupe, V.M., Jansma, E.P., Smit, E.F., Uyl-de Groot, C.A.: Cost effectiveness of treatment with new agents in advanced non-small-cell lung cancer: a systematic review. Pharmacoeconomics. 30(1), 17–34 (2012). https://doi.org/10.2165/11595000-000000000-00000
Siebert, U.: When Should Decision-Analytic Modeling be Used in the Economic Evaluation of Health Care? Springer, Berlin (2003)
Luijn, J.C.v., Loenen, A.C.v., Gribnau, F.W., Leufkens, H.G.: Choice of comparator in active control trials of new drugs. Ann. Pharmacother. 42(11), 1605–1615 (2008). https://doi.org/10.1345/aph.1L115
National Institute for Health and Clinical Excellence: Erlotinib and gefitinib for treating non-small-cell lung cancer that has progressed after prior chemotherapy. https://www.nice.org.uk/guidance/ta374/chapter/1-Guidance (2015). Accessed 20 Nov 2018
Littlewood, C., Ashton, J., Chance-Larsen, K., May, S., Sturrock, B.: The quality of reporting might not reflect the quality of the study: implications for undertaking and appraising a systematic review. J. Man. Manip. Ther. 20(3), 130–134 (2012). https://doi.org/10.1179/2042618611y.0000000013
Desai, P.R., Chandwani, H.S., Rascati, K.L.: Assessing the quality of pharmacoeconomic studies in India. Pharmacoeconomics 30(9), 749–762 (2012)
Acknowledgements
No funding has been received for the conduct of this study and/or preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
CTTN: study rationale and design, literature search, literature selection, quality assessment of studies, interpretation and reflection, writing of the manuscript. FP: literature search, literature selection, quality assessment of studies, revising and reviewing of the manuscript. SS: literature selection, interpretation and reflection, revising and reviewing of the manuscript. BTN: study rationale and design, revising and reviewing the manuscript. IG: quality assessment of studies, interpretation and reflection, providing critical comments, revising and reviewing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest exist in this work. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Location—published year—author | Europe—2016—Vergnenegre [32] | USA—2015—Ting [42] | UK—2015—Khan [33] | Germany—2015—Schremser [34] | Hong Kong—2014—Lee [39] | China—2013—Wang [40] | Europe—2012—Walleser [38] | France—2013—Chouaid [36] | France—2012—Chouaid [43] | Europe—2012—Vergnenègre [37] | China—2017—Fangbing [41] |
|---|---|---|---|---|---|---|---|---|---|---|---|
QHES question (y = full score, p = partial score, n = zero score) | |||||||||||
1. Was the study objective presented in a clear, specific, and measurable manner? | y | y | y | y | y | y | y | y | y | y | y |
2. Were the perspective of the analysis (societal, third-party payer, etc.) and reasons for its selection stated? | p | n | n | p | p | y | y | p | p | p | n |
3. Were variable estimates used in the analysis from the best available source (i.e., randomized control trial - best, expert opinion - worst)? | y | y | y | y | y | p | p | y | y | y | y |
4. If estimates came from a subgroup analysis, were the groups prespecified at the beginning of the study? | y | y | y | y | y | y | y | y | y | y | y |
5. Was uncertainty handled by (1) statistical analysis to address random events, (2) sensitivity analysis to cover a range of assumptions? | y | y | y | y | y | y | y | y | y | n | y |
6. Was incremental analysis performed between alternatives for resources and costs? | y | y | y | y | y | y | y | y | y | y | y |
7. Was the methodology for data abstraction (including the value of health states and other benefits) stated? | p | p | y | y | p | y | p | p | p | n | y |
8. Did the analytic horizon allow time for all relevant and important outcomes? Were benefits and costs that went beyond 1 year discounted (3–5%) and justification given for the discount rate? | y | y | y | y | y | y | y | n | n | y | n |
9. Was the measurement of costs appropriate and the methodology for the estimation of quantities and unit costs clearly described? | y | y | y | y | p | y | n | y | y | y | y |
10. Were the primary outcome measure(s) for the economic evaluation clearly stated and did they include the major short-term? was justification given for the measures/scales used? | y | p | y | y | p | n | y | p | p | n | y |
11. Were the health outcomes measures/scales valid and reliable? If previously tested valid and reliable measures were not available, was justification given for the measures/scales used? | p | p | y | y | y | n | y | y | y | y | n |
12. Were the economic model (including structure), study methods and analysis, and the components of the numerator and denominator displayed in a clear, transparent manner? | y | y | p | y | p | y | p | n | n | y | y |
13. Were the choice of economic model, main assumptions, and limitations of the study stated and justified? | y | y | y | y | y | y | p | n | n | n | y |
14. Did the author(s) explicitly discuss direction and magnitude of potential biases? | n | n | n | n | n | n | n | n | n | n | n |
15. Were the conclusions/recommendations of the study justified and based on the study results? | y | y | y | y | y | y | y | y | y | y | y |
16. Was there a statement disclosing the source of funding for the study? | n | y | y | y | y | y | n | y | y | y | n |
Total score | 83 | 81 | 86 | 92 | 79 | 77 | 69 | 64.5 | 64.5 | 65 | 73 |
Rights and permissions
About this article
Cite this article
Nguyen, C.T.T., Petrelli, F., Scuri, S. et al. A systematic review of pharmacoeconomic evaluations of erlotinib in the first-line treatment of advanced non-small cell lung cancer. Eur J Health Econ 20, 763–777 (2019). https://doi.org/10.1007/s10198-019-01040-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-019-01040-7