Abstract
Objective
To compare the cost-effectiveness of injectable disease-modifying therapies (DMTs) for the first-line treatment of relapsing-remitting multiple sclerosis (RRMS) in Spain.
Methods
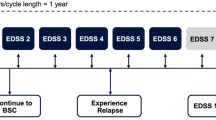
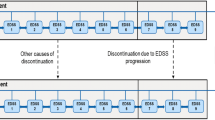
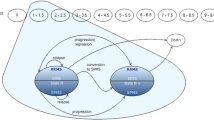
A Markov model was developed to estimate the cost-effectiveness of intramuscular interferon beta-1a (IM IFNβ-1a), subcutaneous interferon beta-1a (SC IFNβ-1a), interferon beta-1b (IFNβ-1b) and glatiramer acetate (GA) relative to best supportive care in a hypothetical cohort of 1,000 RRMS patients in Spain. The model was developed from a societal perspective with a time horizon of 30 years. Natural history and clinical trial data were used to model relapse rates and disease progression. Cost and utility data were obtained from a published survey of multiple sclerosis patients in Spain. The primary outcome measure was cost per quality-adjusted life year (QALY) gained. Univariate and probabilistic sensitivity analyses were performed.
Results
Compared to best supportive care, the base case cost-effectiveness was €168,629 per QALY gained for IM IFNβ-1a, €231,853 per QALY gained for IFNβ-1b, €295,638 per QALY gained for SC IFNβ-1a, and €318,818 per QALY gained for GA. Results were most sensitive to changes in DMT cost, utility values and treatment effect.
Conclusions
In our cost-effectiveness analysis of first-line injectable DMTs in Spain, we found IM IFNβ-1a to be more cost-effective than SC IFNβ-1a, IFNβ-1b or GA. Sensitivity analyses confirmed the robustness of these results.




Similar content being viewed by others
References
Goodin, D.S., Frohman, E.M., Garmany, G.P., et al.: Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for clinical practice guidelines. Neurology 58(2), 169–178 (2002)
National Multiple Sclerosis Society. Multiple sclerosis: just the facts. MS Soc 2010: available at http://nationalmssociety.org (2011). Accessed 13 July 2011
Multiple Sclerosis International Federation. About MS. Available at http://www.msif.org/en/about_ms (2011). Accessed 10 August 2011
Asociacion Espanola de Esclerosis Multiple. Multiple Sclerosis: living with multiple sclerosis. Available at http://www.aedem.info/portal/ (2011). Accessed 10 August 2011
Pozzilli, C., Romano, S., Cannoni, S.: Epidemiology and current treatment of multiple sclerosis in Europe today. J. Rehabil. Res. Dev. 39(2), 175–185 (2002)
Bufill, E., Blesa, R., Galan, I., et al.: Prevalence of multiple sclerosis in the region of Osona, Catalonia, northern Spain. J. Neurol. Neurosurg. Psychiatry 58, 577–581 (1995)
Benito-Leon, J., Martin, E., Vela, L., et al.: Multiple sclerosis in Mostoles, central Spain. Acta Neurol. Scand. 98, 238–242 (1998)
Tola, M., Yugueros, M., Fernandez-Buey, N., et al.: Prevalence of multiple sclerosis in Vallaloid, northern Spain. J. Neurol. 246, 170–174 (1999)
Pugliatti, M., Rosati, G., Carton, H., et al.: The epidemiology of multiple sclerosis in Europe. Eur. J. Neurol. 13, 700–722 (2006)
Ares, B., Prieto, J.M., Lema, M., et al.: Prevalence of multiple sclerosis in Santiago de Compostela (Galicia, Spain). Mult. Scler. 13, 262–264 (2007)
Kobelt, G., Berg, J., Lindgren, P., et al.: Costs and quality of life of multiple sclerosis in Spain. Eur. J. Health Econ. 7(Suppl 2), S65–S74 (2006)
Jacobs, L.D., Cookfair, D.L., Rudick, R.A., et al.: Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann. Neurol. 39(3), 285–294 (1996)
Johnson, K., Brooks, B., Cohen, J., et al.: Copolymer 1 reduces relapse rates and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo-controlled trial. Neurology 45, 1268–1276 (1995)
PRISMS: Randomised double-blind placebo controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 52, 1498–1504 (1998)
The PRISMS Study Group: PRISMS-4: long-term efficacy of interferon beta-1a in relapsing MS. Neurology 56, 1628–1636 (2001)
The IFNB Multiple Sclerosis Group and the University of British Colombia MS/MRI Analysis Group: Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 45, 1277–1285 (1995)
INFB Multiple Sclerosis Study Group: Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. Neurology 43, 655–661 (1993)
Bell, C., Graham, J., Earnshaw, S., et al.: Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J. Manag. Care Pharm. 13(3), 245–261 (2007)
Chilcott, J., McCabe, C., Tappenden, P., et al.: Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. Commentary: evaluating disease modifying treatments in multiple sclerosis. BMJ. 326(7388), 522 (2003). (discussion p 22)
Goldberg, L.D., Edwards, N.C., Fincher, C., et al.: Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J. Manag. Care Pharm. 15(7), 543–555 (2009)
Guo, S., Bozkaya, D., Ward, A., et al.: Treating relapsing multiple sclerosis with subcutaneous versus intramuscular interferon-beta-1a: modelling the clinical and economic implications. Pharmacoeconomics 27(1), 39–53 (2009)
Kobelt, G., Texier-Richard, B., Lindgen, P.: The long-term cost of multiple sclerosis in France and potential changes with disease-modifying interventions. Mult. Scler. 15, 741–751 (2009)
Noyes, K., Bajorska, A., Chappel, A., et al.: Cost-effectiveness of disease-modifying therapy for multiple sclerosis. Neurology 77, 355–363 (2011)
Prosser, L.A., Kuntz, K.M., Bar-Or, A., et al.: Cost-effectiveness of interferon beta-1a, interferon beta-1b, and glatiramer acetate in newly diagnosed non-primary progressive multiple sclerosis. Value Health 7(5), 554–568 (2004)
Nuijten, M., Mittendorf, T.: A health-economic evaluation of disease-modifying drugs for the treatment of relapsing-remitting multiple sclerosis from the German Societal Perspective. Clin. Ther. 32(4), 717–728 (2010)
Sanchez-de la Rosa, R., Sabater, E., Casado, M.A. et al.: Cost-effectiveness analysis of disease modifying drugs (interferons and glatiramer acetate) as first line treatments in remitting-relapsing multiple sclerosis patients. J. Med. Econ. 15(3), 424–433 (2012)
Tappenden, P., McCabe, C., Chilcott, J., et al.: Cost-effectiveness of disease-modifying therapies in the management of multiple sclerosis for the medicare population. Value Health 12(5), 657–665 (2009)
Kurtzke, J.: Rating neurologic impairment in multiple sclerosis: an expanded disability status scale. Neurology 33, 1444–1452 (1983)
Weinshenker, B.G., Bass, B., Rice, G.P., et al.: The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 112(Pt 1), 133–146 (1989)
Earnshaw, S.R., Graham, J., Oleen-Burkey, M.K.: Cost effectiveness of glatiramer acetate and natalizumab in relapsing-remitting multiple sclerosis. Appl. Health Econ. Health Policy 7(2), 91–108 (2009)
Runmarker, B., Andersen, O.: Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Brain 116(Pt 1), 117–134 (1993)
OECD/European Union: “Mortality from all Causes”, in Health at a Glance: Europe 2010, OECD Publishing. (2010). http://dx.doi.org/10.1787/health_glance-2010-en
Broman, T., Andersen, O., Bergmann, L.: Clinical studies on multiple sclerosis. I. Presentation of an incidence material from Gothenburg. Acta Neurol. Scand. 63(1), 6–33 (1981)
Fog, T., Linnemann, F.: The course of multiple sclerosis in 73 cases with computer-designed curves. Acta Neurol Scand Suppl 47, 3–175 (1970)
Patzold, U., Pocklinton, P.: Course of multiple sclerosis: first results of a prospective study carried out of 102 MS patients from 1976–1980. Acta Neurol Scand 65, 248–266 (1982)
Thompson, J.P., Noyes, K., Dorsey, E.R., Schwid, S.R., Holloway, R.G.: Quantitative risk-benefit analysis of natalizumab. Neurology 71(5), 357–364 (2008)
IHS Global Insight: Ex-factory Spanish Prices as of July 2010 (2011)
Spanish Consumer Price Index from the Instituto Nacional de Estadistica
The EuroQol Group: EuroQol—a new facility of the measurement of health-related quality of life. Health Policy 16, 199-208 (1990)
Weinstein, M., O’Brien, B., Hornberger, J., et al.: Principles of good practice for decision analytic modeling in health care evaluation: report of the ISPOR task force on good research practices—modeling studies. Value Health 6(1), 9–17 (2003)
Philips, Z., Bojke, L., Sculpher, M., et al.: Good practice guidelines for decision-analytic modelling in health technology assessment. Pharmacoeconomics 24(4), 355–371 (2006)
Acknowledgments
This research was funded by Biogen Idec.
Conflict of interest
Carole Dembek and Leigh Ann White are employees and shareholders of Biogen Idec. Andrea Szkurhan, Jayson Quach and Nazia Rashid were paid consultants on the study. M.R. Blasco received consulting fees from Biogen Idec for participation in this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dembek, C., White, L.A., Quach, J. et al. Cost-effectiveness of injectable disease-modifying therapies for the treatment of relapsing forms of multiple sclerosis in Spain. Eur J Health Econ 15, 353–362 (2014). https://doi.org/10.1007/s10198-013-0478-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-013-0478-z