Abstract
To characterize primary failure to infliximab and determine the efficacy of switching to tocilizumab in patients with rheumatoid arthritis (RA), we examined 24 RA patients who had started on infliximab therapy (3 mg/kg) as their first biological agent. Nine of the 24 patients were found to be primary nonresponders, defined as patients who had never achieved a 20% clinical improvement according to the American College of Rheumatology criteria (ACR20) during induction therapy. The remaining 15 patients had achieved an ACR20 response to infliximab, without any relapses, for at least the first 14 weeks. A higher baseline health assessment questionnaire score was markedly associated with primary unresponsiveness to infliximab (p = 0.0005). Six of the 9 primary nonresponders showed rapid clearance of infliximab: their trough concentrations of infliximab were under 1 μg/ml. The other 3 were classified as exhibiting the residual type of unresponsiveness, which was defined as unresponsiveness in patients who maintained serum infliximab levels above 1 μg/ml. Human antichimeric antibody was not detected in the rapid-clearance nonresponders. Dose escalation (5 mg/kg) was insufficiently effective. Primary nonresponders to infliximab were started on tocilizumab therapy (8 mg/kg, every 4 weeks), and their responses were assessed after 24 weeks of this second attempt at therapy. All the nonresponders, except for a single rapid-clearance patient, had achieved an ACR20 clinical improvement at the time of assessment. In conclusion, primary nonresponders to infliximab can be classified into rapid-clearance and residual types, based on their trough concentrations of infliximab, but both types of nonresponders seem to benefit from an early decision to discontinue infliximab therapy and switch to tocilizumab.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prognosis of rheumatoid arthritis (RA) has improved dramatically with the development of novel therapeutic strategies targeted at specific cytokines such as tumor necrosis factor-α (TNFα), but we have learned through daily practice that not all RA patients treated with anti-TNFα agents show good therapeutic responses. Trial studies have also shown that approximately 30% of individuals who try these agents fail to achieve a 20% clinical improvement according to the American College of Rheumatology criteria (ACR20) [1–3]. Recent studies have identified two types of lack of efficacy that can bring about the failure of anti-TNFα therapy [4–6]. One is the absence of any clinical response (primary lack of efficacy); the other is the disappearance of an initial favorable response during therapy (secondary loss of efficacy). It has been suggested that switching to an alternative anti-TNFα agent may be less effective in nonresponders who showed a primary lack of efficacy than in those in whom the first anti-TNFα agent was withdrawn due to a secondary loss of efficacy [7–9]. To date, however, we lack reliable guidelines for choosing alternative treatments for individual RA patients who fail to respond to their first anti-TNFα agent [10, 11]. Therapeutic decisions regarding switching therefore depend on individual rheumatologists’ experience, patients’ preferences, and the risks associated with individual drugs.
Encouraging data from recent phase-III trials of tocilizumab, a humanized monoclonal anti-interleukin (IL)-6 receptor (IL-6R) antibody, have led to its approval in Japan, Europe, and the United States for the treatment of patients with moderate to severe RA showing inadequate response to conventional disease-modifying anti-rheumatic drugs (DMARDs) and anti-TNFα agents [12]. Approximately 60–80% of DMARD-resistant patients in the group receiving 8 mg/kg of tocilizumab every 4 weeks achieved an ACR20 at 24 weeks [13–15]. In one large study lasting for 52 weeks, patients in the tocilizumab-treated group had significantly less radiographic progression than those treated with conventional DMARDs [16]. Furthermore, Emery et al. [17] have shown that an ACR20 was achieved at 24 weeks by 50% of patients in the tocilizumab-treated group who had inadequate response to one or more anti-TNFα agents, although the type of failure of anti-TNFα therapy in individual patients was not determined. Kawashiri et al. [18] have reported that tocilizumab induced remarkable clinical responses in secondary nonresponders to anti-TNFα agents. The remaining question is how primary nonresponders to anti-TNFα agents may benefit from a switch to this new biological agent with its different mechanism of action.
The detailed mechanisms underlying primary failure to infliximab, a chimeric anti-TNFα monoclonal antibody, are poorly understood. Since several studies have suggested that favorable therapeutic outcomes depend in part on sufficient exposure to infliximab during the course of therapy [19–22], it appears that the serum concentration of infliximab at the end of a dosing period (trough concentration) may be a useful clue in exploring the mechanisms of primary failure. Here, we report on 9 patients with RA who showed a primary lack of efficacy of infliximab. To identify the responsible mechanisms, we measured serum trough concentrations of infliximab and examined the formation of human antichimeric antibody (HACA) in sera. We also assessed the efficacy of switching to tocilizumab in primary infliximab-nonresponders, based on the ACR improvement criteria and the European League Against Rheumatism (EULAR) response criteria.
Patients, materials, and methods
Patients, study protocol, and evaluation
From June 2009 to June 2010, we initiated infliximab therapy in 24 patients with RA at Kumamoto Saishunsou National Hospital, who had never previously received any biological agent. All participants fulfilled the 1987 ACR criteria for a diagnosis of RA. Eligibility for infliximab therapy was determined according to the revised guidelines officially approved by the Japan College of Rheumatology (JCR) in 2008: namely, a patient is eligible for consideration of infliximab therapy if RA is inadequately controlled despite treatment for at least 3 months with standard doses of conventional DMARDs, usually methotrexate (MTX, more than 6 mg/week); inadequate control is defined as the presence of 6 or more tender joints, 6 or more swollen joints, and either an erythrocyte sedimentation rate (ESR) equal to or higher than 28 mm/h or C-reactive protein (CRP) level equal to or higher than 2 mg/dl. In addition, patients with radiographic progression, defined as an increase of the total Sharp score by more than 10 points per year, and those with a disease activity score for 28 joints (DAS28 score) equal to or greater than 3.2, were also considered eligible for infliximab therapy.
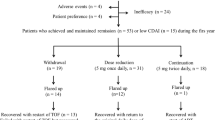
Patients were scheduled to receive a 3-infusion induction regimen, consisting of intravenous infusions of infliximab (3 mg/kg) at weeks 0, 2, and 6. During infliximab therapy, 8 mg/week of MTX and 5 mg/week of folic acid were given concomitantly. Patients who were receiving prednisolone at the start of infliximab therapy continued to receive the same dose of prednisolone concomitantly throughout the period of infliximab therapy (2 patients receiving 10 mg/day and 2 receiving 5 mg/day). Clinical responses were evaluated according to the ACR improvement criteria and the EULAR response criteria. Primary nonresponders were defined as patients who never achieved an ACR20 response during this therapy. The type of primary failure in each patient was determined based on trough serum concentrations of infliximab immediately before each infusion. The residual type of unresponsiveness was defined as unresponsiveness in patients who maintained serum infliximab levels above 1 μg/ml; a lack of efficacy due to rapid clearance was defined as unresponsiveness in patients whose trough concentrations of infliximab were under 1 μg/ml. The primary nonresponders, with two exceptions, stopped infliximab therapy before the fourth infusion; the two exceptions were both patients with rapid clearance (cases 1 and 4) for whom we changed the dose to 5 mg/kg at the fourth infusion (dose escalation) and determined serum trough concentrations and clinical responses 8 weeks later. Patients who had achieved and maintained an ACR20 response by week 14 started maintenance therapy with 3 mg/kg of infliximab every 8 weeks.
All patients exhibiting primary lack of efficacy of infliximab were switched to tocilizumab therapy (intravenous infusion of 8 mg/kg every 4 weeks for 24 weeks). MTX (8 mg/week) and 5 mg/week of folic acid were given concomitantly. Clinical responses were evaluated according to the ACR improvement criteria and the EULAR response criteria. Disease activity was measured according to the DAS28 score, which was calculated based on a swollen joint count in the 28 joints (SJC28), a tender joint count in the 28 joints (TJC28), ESR, and the patients’ own global evaluation of their general health on a visual analog scale (VAS, scale of 0–100). Serum CRP levels, Health Assessment Questionnaire (HAQ) score, SJC66, and TJC68 were also recorded.
The ethics committee of Kumamoto Saishunsou National Hospital approved the protocol for this study, and written informed consent was obtained from all patients.
Infliximab serum assay and HACA detection
Blood samples were collected immediately prior to each infusion. Serum trough concentrations of infliximab were measured through an enzyme-linked immunosorbent assay (ELISA) using a monoclonal antibody specific to the TNFα binding site [23]. The captured infliximab was detected by a biotinylated monoclonal antibody specific for an epitope in its variable region. The lowest level that could reliably be detected was 0.1 μg/ml. HACA was measured using a modified version of the ELISA method originally reported by LoBuglio et al. [24], when the lowest serum concentrations of infliximab were below the detectable limit of the assays.
Measurements of tocilizumab
Serum trough concentrations of tocilizumab were measured through an ELISA using a mouse anti-IL-6R monoclonal antibody (MT-18) in combination with human soluble IL-6R [25]. The captured tocilizumab was detected by a biotinylated monoclonal antibody specific for an epitope in its variable region. The lowest concentration that could reliably be detected was 1.0 μg/ml. This assay was performed at SRL (Tachikawa, Japan).
Measurements of anti-cyclic citrullinated peptide antibodies (anti-CCP Abs) and IgM rheumatoid factor (RF)
Sera that had been collected at the start and end of each course of therapy and stored at −80°C were examined for concentrations of anti-CCP Abs and IgM RF. Anti-CCP Abs were measured using a commercially available ELISA kit (anti-CCP2 assay; Axis-Shield Diagnostic, Dundee, Scotland) according to the manufacturer’s instructions. The concentrations of anti-CCP Abs were estimated by interpolating from a standard dose–response curve. A serum sample was considered to be anti-CCP-positive if its absorbance value was greater than 4.6 U/ml. Anti-CCP Abs were measured at SRL. IgM RF was measured by means of nephelometry; a serum sample was considered to be positive if the concentration was higher than the cut-off value of the kit (15 IU/ml).
Statistical analysis
In analyses of categorical variables, levels of significance were determined by means of the χ2test, using 2 × 2 contingency tables. If cell values were less than 5, Fisher’s exact probability test was used. Continuous variables were assessed using the Mann–Whitney U-test. For all tests, probability (p) values of < 0.05 were considered to indicate statistical significance. All calculations were performed using Excel Statistical Analysis 2008 (SSRI, Tokyo, Japan).
Results
Baseline characteristics of RA patients showing a primary lack of efficacy
Among the 24 patients who received infliximab therapy, 9 were characterized by a primary lack of efficacy of infliximab. During the induction regimen of infliximab, these primary nonresponders exhibited no response and never achieved an ACR20 response. The remaining 15 patients did achieve an ACR20 response to infliximab (3 patients with ACR20; 7 with ACR50; and 5 with ACR70) and had no relapses for at least the first 14 weeks. When clinical responses to infliximab were evaluated according to the EULAR response criteria, 15 patients had achieved a good or moderate response (7 patients with a good response and 8 with a moderate response). One patient who was classified as a nonresponder according to the ACR criteria had achieved a moderate response according to the EULAR response criteria, and one patient who was classified into the responder group according to the ACR criteria had failed to achieve a good or moderate EULAR response. As shown in Table 1, males were more likely to show a primary lack of efficacy (p = 0.047). A higher baseline HAQ score was markedly associated with primary unresponsiveness to infliximab therapy (p = 0.0005). Initial levels of DAS28 and ESR were also significantly higher in the primary nonresponders compared with the responder group (p = 0.04).
Demographic and clinical data of the primary nonresponders at the start of infliximab therapy are shown in Table 2. There were five cases of early RA (3–14 months) that had presented with abrupt-onset acute polyarthritis (cases 2–6); the other 4 were long-standing RA cases (3–10 years) showing a progressive pattern of the clinical course (cases 1 and 7–9). Before the introduction of infliximab therapy, all nonresponders had received 8 mg/week of MTX and 2 patients had also received 10 mg/day of prednisolone; nevertheless, their disease activity had remained high and they had complained of intense joint pain, diffuse swelling, and difficulty in performing activities of daily living.
Characterization of primary lack of efficacy based on trough concentrations of infliximab
In the nonresponders, the number of joints involved actually increased during induction therapy with infliximab (Table 3). Indices of disease activity and disability had deteriorated. In 6 patients (cases 1–6), trough concentrations of serum infliximab were below the therapeutic level (1 μg/ml) (Fig. 1). Among these, cases 1 and 4 had received a dose escalation at the fourth infusion (5 mg/kg of infliximab at week 14), but they nevertheless showed undetectably low trough concentrations (less than 0.1 μg/ml). HACA was not detected in any of the patients whose lowest serum concentrations of infliximab were below the detectable limit of the assays. In contrast, the other 3 patients (cases 7–9) had maintained serum infliximab levels sufficiently high to produce therapeutic effects. As shown in Fig. 1, approximately 80% of the responders maintained serum concentrations of infliximab above 1 μg/ml, even immediately before the 4th infusion. We concluded that all nonresponders were characterized by a primary lack of efficacy of infliximab. Rapid clearance was the main cause of failure in cases 1–6, while the other cases were classified as the residual type. We decided to switch the patients from infliximab to tocilizumab.
Trough serum concentrations of infliximab in the responder group and the nonresponder group. Blood samples were collected immediately before the 2nd infusion (open circle), the 3rd infusion (closed circles), or the 4th infusion (closed squares) of infliximab. Nine patients in the responder group had available data. The broken line indicates the lower limit of the therapeutic level (1 μg/ml)
Therapeutic effects of switching to tocilizumab in primary nonresponders to infliximab
The primary nonresponders to infliximab started tocilizumab therapy in combination with MTX. Twenty-four weeks after making this switch, all the nonresponders, except for case 6, had achieved a good or moderate response as defined by the EULAR criteria and a 20, 50, or 70% clinical improvement as defined by the ACR criteria, as shown in Table 4. Four patients had achieved clinical remission (cases 3–5 and 9). Case 6 had transiently achieved an ACR20 response at week 16 but had subsequently relapsed. At week 24, this patient’s trough concentration of serum tocilizumab was still detectable (2.3 μg/ml) and no anti-tocilizumab antibody was observed.
Discussion
In 6 patients in the present study (cases 1–6), unresponsiveness to infliximab therapy was characterized by a primary lack of efficacy due to rapid clearance. These patients failed to maintain serum concentrations of infliximab above 1 μg/ml during 14 weeks of induction therapy. Several groups have shown that trough serum concentrations of infliximab are related to clinical improvement in RA and that the lower limit of the therapeutic level is approximately 1 μg/ml [19–22]. Furthermore, we have shown that, during 14 weeks of infliximab therapy at 3 mg/kg via intravenous infusion, most good and moderate responders exhibited trough concentrations greater than 1 μg/ml [4]. The drug’s rapid clearance may be explained by high levels of TNFα production, since it is possible that TNFα-infliximab complexes are eliminated from the circulation at a higher rate than unbound infliximab molecules are [4, 21]. A revival of TNFα-producing cells and a subsequent overproduction of TNFα during infliximab therapy may induce a rapid clearance of infliximab from the body. For patients with high disease activity, therefore, we need to pay special attention to trough concentrations of infliximab. It has been reported that HACA formation may possibly alter the pharmacokinetics of infliximab [23], but HACA was not detected in any patients who had shown a rapid clearance of infliximab in the present study, suggesting that the rapid clearance observed here was not due to the formation of anti-infliximab antibodies. Given that a dose–response relationship exists, dose escalation may be beneficial in some cases of this type. Two randomized, double-blind studies (the RISING and START studies) have demonstrated that a considerable proportion of patients showed better clinical responses after receiving dose escalation of infliximab, without an increased risk of adverse events [19, 22]. In the present study, two patients with rapid clearance received a dose escalation (5 mg/kg) at week 14, but trough concentrations were still undetectably low and no clinical improvement was observed. In such cases, shortening the dosing interval could be an alternative means of improving clinical responses to infliximab therapy [20, 21]. Based on a randomized, double-blind study, however, Pavelka et al. [26] have indicated that dose escalation did not improve therapeutic efficacy for RA. In a systematic review and meta-analysis of randomized, controlled trials for two licensed anti-TNF antibodies, Bongartz et al. [27] reported a dose-dependent increased risk of malignancies; nevertheless, a recent systematic review of data from the United States, Canadian, Swedish, German, Spanish, and British registries and data from long-term, open-label extension studies has shown no increase in the overall risk of malignancy in patients exposed to anti-TNFα therapy [28]. Longitudinal controlled studies may be needed to more precisely determine the efficacy and safety of dose escalation in infliximab therapy for RA.
In the present study, we also observed 3 patients showing the residual type of unresponsiveness to infliximab therapy (cases 7–9). Throughout the course of infliximab therapy, these patients maintained sufficient levels of serum infliximab, yet still exhibited a primary lack of efficacy. This type of primary unresponsiveness has rarely been documented. In such cases, TNFα may not be the main factor driving the inflammatory process in RA, or alternative inflammatory pathways, such as IL-6 signaling, may be utilized to evade the inhibitory effects of the anti-TNFα agent. Either way, it seems rational to discontinue infliximab therapy in such cases. For primary nonresponders to infliximab without rapid clearance, it may be helpful and cost-effective to switch to tocilizumab, because these two biological agents target different molecules in the inflammatory cascade of RA.
In the present study, 37.5% of RA patients had never achieved an ACR20 response during induction therapy with infliximab; a good or moderate EULAR response was achieved in 62.5% of patients. This rate of clinical improvement in response to infliximab was lower than that seen in our previous study, in which 15 patients (83.3%) had achieved a good or moderate response by week 14 of therapy and only 3 patients were found to be nonresponders [4]. Retrospective clinical studies by an RA management group in Japan (the RECONFIRM and RECONFIRM II studies) have shown that, by week 22 of infliximab therapy, 84.5% of patients achieved a good or moderate EULAR response [29, 30]. The RISING study, also conducted in Japan, has reported that an ACR20 response was achieved in 75.8% of patients by week 54 [22]. In Western countries, however, a randomized controlled trial has shown that only 62.4% of MTX-naïve patients with early RA achieved an ACR20 clinical improvement by week 54 [31]. Using results from the British registry, Hyrich et al. [32] have reported that 33% of RA patients exhibited no EULAR response to infliximab therapy after 6 months. Likewise, Abe et al. have reported that the ACR20 improvement rate at week 14 was only 61.2% in a Japanese clinical trial [33]. These figures are in keeping with the present data. A considerable number of patients participating in this study were referred to our hospital for management of RA because they showed increases in joint involvement despite MTX therapy or because their RA presented with abrupt, acute polyarthritis; both of these are less common types of RA. Considering our small sample size and relatively short period of observation, we cannot exclude the possibility that this referral bias may have influenced the response rate to infliximab.
A considerable number of observational studies have shown that switching between anti-TNFα agents is beneficial for RA patients; however, the probability of achieving a sufficient clinical response and the average magnitude of response associated with sequential anti-TNFα treatments are lower than those for first-time anti-TNFα treatments [34–36]. Data from the British registry show that the reasons for failure of the first anti-TNFα drug can recur with the new drug after switching, and investigators have identified a group of patients with multiple anti-TNFα failures [37]. In a report based on the Swedish registry, the therapeutic responses achieved by patients who switched between anti-TNFα agents were limited compared with those of anti-TNFα-naïve patients [38]. Similar conclusions have been drawn based on the Spanish registry: the response to a second or third anti-TNFα agent was much smaller than the response to the first one [39]. Furthermore, it has not yet been established whether the type of failure that motivated the switch from the first anti-TNFα agent, such as primary lack of efficacy, secondary loss of efficacy, or adverse effects, influences the response to the new drug [10, 11]. In the British, Swedish, and Spanish registries, however, RA patients who had stopped a first anti-TNFα agent because of lack of efficacy showed less extensive improvements in response to a second agent than those who had discontinued due to adverse effects [37, 38, 40]. In a recent review of the literature, Scrivo et al. [41] have suggested that RA patients who withdrew a first anti-TNFα agent due to secondary failure or adverse effects may be successfully treated with another anti-TNFα agent, whereas patients with primary failure to a first anti-TNFα agent may find that a different type of biological agent is the best alternative.
In a previous study, we reported that for patients who showed a rapid clearance of infliximab, increased use of prednisolone or MTX was beneficial to achieve sufficient clinical responses [4]. At the time of that study, 6 mg/week of MTX was considered the optimal dosage for RA patients in Japan, and it was recommended that the maximum dosage be restricted to 8 mg/week. Very recently, however, dosages of up to 16 mg/week were approved in Japan; accordingly, increasing the dosage of MTX may be one therapeutic option for RA patients with rapid clearance of infliximab. Since the revised 2008 JCR guidelines for the use of infliximab have warned that prednisolone contributes to an increased risk of serious infection during infliximab therapy, increasing the dosage of prednisolone is not feasible in our current practice. We have also demonstrated that tacrolimus, a suppressor of the activation of antigen-specific T cells, induced good clinical responses for RA patients who were characterized by the primary lack of efficacy for infliximab, regardless of the presence or absence of rapid clearance [4]. Besides targeting specific cytokines such as TNFα and IL-6, it may be required to focus on events upstream in the inflammatory cascade, such as T-cell activation.
In the present study, no serious adverse events were observed during the 24-week courses of tocilizumab therapy; nevertheless, several issues related to the safety of tocilizumab are worth mentioning here. The 2010 revised version of the JCR official guidelines for the use of tocilizumab has recommended that, when it is used for RA patients with multiple risk factors for serious infections, including advanced age, pulmonary comorbidities, prednisolone use (more than 5 mg/day), long disease duration (more than 10 years), and Steinbrocker class 3 or 4, its use must be preceded by a careful assessment of the predictable risks juxtaposed with the foreseeable benefits. In addition, rheumatologists should be aware that tocilizumab can mask signs of infection such as fever and general fatigue and can suppress elevations of inflammatory markers such as CRP and ESR, thereby leading to delay in the diagnosis of infectious diseases.
In the present study, the primary nonresponders were classified into two groups, the rapid-clearance type and the residual type, according to their trough concentrations of infliximab. Both types of primary nonresponders seem to benefit from switching to tocilizumab. Further research on the therapeutic efficacy of switching to a biological agent of a different class may offer guidance for individual RA patients with primary failure to infliximab when discontinuation of this therapy is under consideration.
References
Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–602.
Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–9.
Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45.
Mori S. A relationship between pharmacokinetics (PK) and the efficacy of infliximab for patients with rheumatoid arthritis: characterization of infliximab-resistant cases and PK-based modified therapy. Mod Rheumatol. 2007;17:83–91.
van Vollenhoven RF. Switching between anti-tumour necrosis factors: trying to get a handle on a complex issue. Ann Rheum Dis. 2007;66:849–51.
Buch MH, Bingham SJ, Bryer D, Emery P. Long-term infliximab treatment in rheumatoid arthritis: subsequent outcome of initial responders. Rheumatology (Oxford). 2007;46:1153–6.
Matsuno H. Etanercept response in patients with rheumatoid arthritis after secondary loss of efficacy of infliximab. Mod Rheumatol. 2010;20:561–5.
Buch MH, Bingham SJ, Bejarano V, Bryer D, White J, Reece R, et al. Therapy of patients with rheumatoid arthritis: outcome of infliximab failures switched to etanercept. Arthritis Rheum. 2007;57:448–53.
Bennett AN, Peterson P, Zain A, Grumley J, Panayi G, Kirkham B. Adalimumab in clinical practice. Outcome in 70 rheumatoid arthritis patients, including comparison of patients with and without previous anti-TNF exposure. Rheumatology (Oxford). 2005;44:1026–31.
Buch MH. Sequential use of biologic therapy in rheumatoid arthritis. Curr Opin Rheumatol. 2010;22:321–9.
Villeneuve E, Haraoui B. To switch or to change class-the biologic dilemma in rheumatoid arthritis. Nat Rev Rheumatol. 2010;6:301–5.
Hushaw LL, Sawaqed R, Sweis G, Reigle J, Gopal A, Brandt D, et al. Critical appraisal of tocilizumab in the treatment of moderate to severe rheumatoid arthritis. Ther Clin Risk Manag. 2010;6:143–52.
Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19:12–9.
Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–80.
Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–97.
Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an X-ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–7.
Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–23.
Kawashiri SY, Kawakami A, Iwamoto N, Fujikawa K, Aramaki T, Tamai M, et al. Switching to the anti-interleukin-6 receptor antibody tocilizumab in rheumatoid arthritis patients refractory to antitumor necrosis factor biologics. Mod Rheumatol. 2010;20:40–5.
Rahman MU, Strusberg I, Geusens P, Berman A, Yocum D, Baker D, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233–8.
St Clair EW, Wagner CL, Fasanmade AA, Wang B, Schaible T, Kavanaugh A, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451–9.
Wolbink GJ, Voskuyl AE, Lems WF, de Groot E, Nurmohamed MT, Tak PP, et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:704–7.
Takeuchi T, Miyasaka N, Inoue K, Abe T, Koike T. Impact of trough serum level on radiographic and clinical response to infliximab plus methotrexate in patients with rheumatoid arthritis: results from the RISING study. Mod Rheumatol. 2009;19:478–87.
Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–63.
LoBuglio AF, Wheeler RH, Trang J, Haynes A, Rogers K, Harvey EB, et al. Mouse/human chimeric monoclonal antibody in man: kinetics and immune response. Proc Natl Acad Sci USA. 1989;86:4220–4.
Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–64.
Pavelka K, Jarosova K, Suchy D, Senolt L, Chroust K, Dusek L, et al. Increasing the infliximab dose in rheumatoid arthritis patients: a randomised, double blind study failed to confirm its efficacy. Ann Rheum Dis. 2009;68:1285–9.
Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85.
Nam JL, Winthrop KL, van Vollenhoven RF, Pavelka K, Valesini G, Hensor EM, et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Ann Rheum Dis. 2010;69:976–86.
Yamanaka H, Tanaka Y, Sekiguchi N, Inoue E, Saito K, Kameda H, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan (RECONFIRM). Mod Rheumatol. 2007;17:28–32.
Tanaka Y, Takeuchi T, Inoue E, Saito K, Sekiguchi N, Sato E, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan: one-year clinical outcomes (RECONFIRM-2). Mod Rheumatol. 2008;18:146–52.
St Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–43.
Hyrich KL, Watson KD, Silman AJ, Symmons DP. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford). 2006;45:1558–65.
Abe T, Takeuchi T, Miyasaka N, Hashimoto H, Kondo H, Ichikawa Y, et al. A multicenter, double-blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. J Rheumatol. 2006;33:37–44.
Lloyd S, Bujkiewicz S, Wailoo AJ, Sutton AJ, Scott D. The effectiveness of anti-TNF-alpha therapies when used sequentially in rheumatoid arthritis patients: a systematic review and meta-analysis. Rheumatology (Oxford). 2010;49:2312–21.
Rubbert-Roth A, Finckh A Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther 2009; 11 Suppl 1:S1
Alivernini S, Laria A, Gremese E, Zoli A, Ferraccioli G. ACR70-disease activity score remission achievement from switches between all the available biological agents in rheumatoid arthritis: a systematic review of the literature. Arthritis Res Ther. 2009;11:R163.
Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum. 2007;56:13–20.
Karlsson JA, Kristensen LE, Kapetanovic MC, Gulfe A, Saxne T, Geborek P. Treatment response to a second or third TNF-inhibitor in RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology (Oxford). 2008;47:507–13.
Navarro-Sarabia F, Ruiz-Montesinos D, Hernandez B, Navarro-Compan V, Marsal S, Barcelo M et al. DAS-28-based EULAR response and HAQ improvement in rheumatoid arthritis patients switching between TNF antagonists. BMC Musculoskelet Disord. 2009;10:91.
Gomez-Reino JJ, Carmona L. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
Scrivo R, Conti F, Spinelli FR, Truglia S, Magrini L, Di Franco M, et al. Switching between TNFalpha antagonists in rheumatoid arthritis: personal experience and review of the literature. Reumatismo. 2009;61:107–17.
Acknowledgments
This study was supported by research funds from the National Hospital Organization (NHO), Japan.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mori, S., Ueki, Y. Primary lack of efficacy of infliximab therapy for rheumatoid arthritis: pharmacokinetic characterization and assessment of switching to tocilizumab. Mod Rheumatol 21, 628–636 (2011). https://doi.org/10.1007/s10165-011-0460-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10165-011-0460-5