Abstract
To evaluate the prevalence of past infection with hepatitis B virus (HBV) in patients with rheumatoid arthritis (RA) and the incidence of its reactivation under treatment with biological and/or nonbiological disease-modifying antirheumatic drugs (DMARDs), 239 patients receiving DMARD therapy were consecutively enrolled and tested for HBV-DNA, using a real-time polymerase chain reaction assay, HBV serology including hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (anti-HBc), and serum levels of aminotransferase. Data prior to DMARD therapy and during follow-up were examined by reviewing medical records. Two patients (0.8%) were positive for HBsAg at the start of therapy. Sixty patients (25.1%) showed HBsAg-negative and anti-HBc-positive serology indicative of past HBV infection. Among these 60 patients, 2 patients (3.3%) experienced reactivation of viral replication (<2.1 log copies/ml) during DMARD therapy. One had been receiving tacrolimus, prednisolone, and methotrexate (MTX); the other had been treated with adalimumab, prednisolone, and MTX. Their serum aminotransferase levels remained normal, and HBsAg was negative. Ten weeks after reactivation of viral replication had been noted, the HBV-DNA titer in the former patient had increased to 2.9 log copies/ml, and HBsAg and hepatitis B e antigen had become weakly positive. In contrast, the latter patient had become negative for viral DNA without any antiviral prophylaxis. In conclusion, the use of biological and nonbiological DMARDs is relatively safe in most RA patients with past HBV infection, even when no anti-HBV prophylaxis is administered. Considering the high prevalence of past infection in RA patients and the high cost of prophylaxis against HBV reactivation, universal prophylaxis is impractical. Regular monitoring of serum viral DNA seems to be the most rational approach to preventing the development of clinically apparent hepatitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic, inflammatory disorder characterized by uncontrolled synovial proliferation in multiple joints. Most patients, if left untreated or inadequately treated, suffer from relentless progressive polyarthritis, causing bone erosion, joint destruction and deformity, and disability, with resultant deterioration in quality of life. Over the past decade, however, the treatment of RA has dramatically changed. It is now well established that when therapy with disease-modifying antirheumatic drugs (DMARDs) is instituted within a few months of disease onset, clinical outcomes are markedly improved [1, 2]. Synthetic DMARDs have been in use for several decades; among these, methotrexate (MTX) is now the most commonly utilized as the first-line DMARD of choice. Further, the emergence of innovative biological agents that target specific molecules and pathways in the immune system has strikingly changed the course of RA and outcomes for patients and society. The use of these biological and nonbiological DMARDs is, nevertheless, limited because they are associated with an increased risk of serious infectious complications, especially those caused by Mycobacterium tuberculosis, atypical mycobacteria, Pneumocystis jirovecii, and other opportunistic bacterial and fungal infections [3–5]. In addition, a number of chronic viral infections can be reactivated during immunosuppressive therapy for rheumatic disease [6].
Reactivation of hepatitis B virus (HBV) replication is a well-recognized complication in patients receiving short-term chemotherapy for malignancies or long-term immunosuppressive therapy after transplantation [7]. HBV reactivation has also been noted in patients treated with synthetic DMARDs, biological agents, and/or high-dose prednisolone for rheumatic disease [8]. Scientific organizations and health authorities around the world have proposed various recommendations for managing patients with chronic HBV infection [9], especially those undergoing immunosuppressive therapy [10, 11]. Rheumatology societies have also published guidelines regarding the use of biological and nonbiological DMARDs in patients with chronic HBV infection [1]; however, more specific consensus guidelines/recommendations on screening practices for HBV infection may be required prior to initiating immunosuppressive therapy for rheumatic disease [12, 13]. Calabrese et al. [8] have recommended screening for HBV markers, including hepatitis B surface antigen (HBsAg), antibody against HBsAg (anti-HBs), and antibody against hepatitis B core antigen (anti-HBc), for all patients with rheumatic diseases requiring immunosuppressive agents that have the potential to induce HBV reactivation.
Patients found to be HBsAg-positive are considered to have a current HBV infection; for these patients, who can include active and inactive carriers, antiviral prophylaxis is necessary before starting immunosuppressive therapy [10, 11]. Patients who are positive for anti-HBc and negative for HBsAg are considered to have had a past HBV infection; these patients may be occult carriers; that is, HBsAg-negative individuals with a long-lasting persistence of viral genomes in the liver tissue and/or serum at very low levels [14, 15]. Currently, all anti-HBc-positive/HBsAg-negative individuals are regarded as potential occult carriers. In addition, occult infection is sometimes found in patients without any serological HBV markers [16]. Data regarding the prevalence of occult infection and the incidence of its reactivation in RA patients under treatment with biological and/or nonbiological DMARDs are still limited and somewhat controversial [17–22]. The optimal protocol for treating such patients is therefore unclear.
To address this issue, I consecutively enrolled 239 RA patients who had been treated with biological and/or nonbiological DMARDs and determined their serum levels of HBV-DNA, status of serological HBV markers, and levels of serum aminotransferase. Data prior to and during DMARD therapy were examined by reviewing medical records.
Patients, materials, and methods
Patients
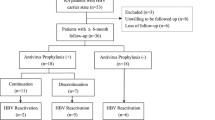
In October and November of 2010, 239 Japanese patients under treatment with biological and/or nonbiological DMARDs for RA were consecutively enrolled at our outpatient clinic. All participants fulfilled the 1987 American College of Rheumatology (ACR) criteria for the diagnosis of RA. Serum levels of HBV-DNA, alanine aminotransferase (ALT), and aspartate aminotransferase (AST), as well as the status of HBsAg and anti-HBc, were determined at enrollment. Data on serological HBV markers and serum ALT and AST levels prior to DMARD therapy and during follow-up (at each visit, i.e., once every 2–3 months) were examined by reviewing the patients’ medical records. The ethics committee of our hospital approved the protocol for this study, and informed consent was obtained from all patients.
Quantification of HBV-DNA and detection of serological HBV markers
HBV-DNA quantification was performed at SRL (Tachikawa, Tokyo, Japan) by real-time polymerase chain reaction (PCR) assay (COBAS AmpliPrep/COBAS TaqMan HBV Test version 2; Roche Diagnostics Japan, Tokyo, Japan). The detection threshold was 19 IU/ml (2.0 log copies/ml) when serum was used as a specimen. The quantifiable range of this assay was 2.1–9.0 log copies/ml. Serological HBV markers (HBsAg and anti-HBc) were all detected using chemiluminescence immunoassays (ARCHITECT System; Abbott Japan, Tokyo, Japan).
Statistical analysis
In analyses of categorical variables, levels of significance were determined by means of the χ2test, using 2 × 2 contingency tables. Continuous variables were assessed using the Mann–Whitney U-test. For all tests, probability values (p values) of <0.05 were considered to indicate statistical significance. All calculations were performed using Excel Statistical Analysis 2008 (SSRI, Tokyo, Japan).
Results
Among the 239 participants, 2 patients (0.8%) were positive for HBsAg when DMARD therapy was first introduced; these patients had been diagnosed as inactive HBV carriers and had been started on anti-HBV prophylaxis with entecavir (0.5 mg/day), based on the recommendation of a hepatologist prior to the commencement of anti-RA therapy. Entecavir was continued during the DMARD therapy (one patient receiving 8 mg/week of MTX and additional tacrolimus; the other receiving 8 mg/week of MTX and additional etanercept), and no reactivation of viral replication was observed, as evidenced by the absence of any increases in ALT/AST levels or HBV-DNA titer. The other patients (n = 237) tested negative for HBsAg at the start of DMARD therapy, and were classified into two groups according to their baseline anti-HBc status (Table 1); namely, an HBsAg-negative/anti-HBc-positive group (individuals with past HBV infection, n = 60, 25.1% of all participants) and the HBsAg-negative/anti-HBc-negative group (n = 177, 74.1%). No change in anti-HBc status was seen in either patient group during follow-up. These patients did not receive any antiviral prophylaxis during anti-RA therapy. At the time of enrollment, the median age of the anti-HBc-positive group was significantly greater than that of the anti-HBc-negative group (73 vs. 62 years, p = 0.0002). There were no significant differences in serum levels of AST or ALT between the groups. The serum AST level was more than twice the upper limit of the normal range in 1 patient (76 IU/l, 1.7%) in the anti-HBc-positive group and 3 patients (73, 75, and 87 IU/l, 1.7%) in the HBc-negative group; no patients showed a twofold or greater increase in serum ALT levels as compared with the upper limit of normal. Only 1 patient in the anti-HBc-negative group tested positive for anti-hepatitis C virus antibody.
With the real-time PCR assay, HBV-DNA was not detected in the sera of any anti-HBc-negative patients. In the anti-HBc-positive group, however, 2 patients (3.3%) tested positive for serum HBV DNA, but the titers in these patients were very low (<2.1 log copies/ml). Both were negative for HBsAg, and their serum levels of AST and ALT were within the normal ranges (cases 1 and 2; Table 2). One year previously, case 1 had been treated with MTX (8 mg/week), but her RA was not adequately controlled. At that time, the patient had tested negative for serum HBV DNA. Nine months before enrollment in the present study, she had been treated with high-dose prednisolone (40 mg/day for 10 days, orally) for minimal-change nephrotic syndrome. The prednisolone was then tapered off to 5 mg/day. Subsequently, this patient was restarted on anti-RA therapy with MTX (8 mg/week), prednisolone (5 mg/day), and tacrolimus (1 mg/day). Before enrollment, the patient had never shown positive results for serum HBsAg or abnormal liver function. Whether her high-dose use of prednisolone for a short period may have been associated with the viral reactivation observed was not clear. Case 2 had previously been treated unsuccessfully with anti-tumor necrosis factor α (anti-TNFα) therapy with infliximab (48 months) and etanercept (4 months); at the time of enrollment, she had been receiving therapy consisting of adalimumab, MTX (6 mg/week), and prednisolone (5 mg/day) for 15 months. It is uncertain when HBV DNA appeared in this patient’s serum, as no increases in serum levels of AST or ALT were observed in regular checkups during anti-RA therapy. In addition, no positive serology for HBsAg was observed throughout this period.
Two months after the viral reactivation had been noted in case 1, the HBV-DNA titer had increased (2.8 log copies/ml), though HBsAg was still negative and serum levels of aminotransferase remained within the normal range. Two weeks after that time, HBsAg and HBeAg became weakly positive (0.14 IU/ml and 2.8 S/CO, respectively) and the HBV-DNA titer was 2.9 log copies/ml. No abnormal liver function was observed. The patient was started on anti-HBV prophylaxis with entecavir. In contrast, in case 2, HBV DNA spontaneously disappeared from the patient’s sera.
Discussion
The prevalence of past HBV infection was 25.1% among the RA patients enrolled in the present study. During biological and/or nonbiological DMARD therapy without anti-HBV prophylaxis, viral DNA reappeared in the serum (<2.1 log copies/ml) in 3.3% of the patients with past HBV infection (2 patients), though serum aminotransferase levels were within the normal range and HBsAg was negative. Two months after the reappearance of viral DNA in the serum, the HBV-DNA titer in one patient had increased to 2.8 log copies/ml, whereas the other patient had become negative for viral DNA without any prophylaxis. These findings suggest that the use of biological and nonbiological DMARDs is relatively safe in most RA patients previously exposed to HBV, even when no anti-HBV prophylaxis is administered. Nevertheless, regular monitoring of viremia is desirable to prevent the development of clinically apparent hepatitis.
Most recent reports from European countries have shown that anti-TNFα therapy appears to be quite safe for patients with rheumatic diseases and past HBV infection, because no reactivation of HBV replication with viral load increases was found even in the absence of antiviral prophylaxis [18–20]. In these studies, the detection threshold of HBV-DNA ranged from 1.7 to 2.5 log copies/ml, the number of patients with past HBV infection ranged from 19 to 67, and the mean follow-up period ranged from 12 to 43 months. A prospective study for Japanese patients with RA and resolved HBV infection has shown that without any anti-HBV prophylaxis, viral reactivation occurred in only one out of 45 patients (2.2%) during immunosuppressive therapy with conventional DMARDs and/or anti-TNFα agents for a mean period of 23 months (range 12–32 months) [22]. In the same study, significant decreases in anti-HBs levels were observed in patients who had received anti-TNFα agents, especially those with low anti-HBs titers at baseline; however, no reactivation of HBV replication was found in this patient population. In the present study, 31 patients with past HBV infection had received anti-TNFα therapy for a median period of 12 months without antiviral prophylaxis. Among these patients, only one patient (3.2%) showed a slight increase in HBV-DNA titer; 2 months after this increase, however, viral DNA had disappeared without any prophylaxis. Antiviral prophylaxis may not be routinely necessary for patients with past HBV infection who are scheduled to receive anti-TNFα therapy, if virological and clinical follow-up is conducted regularly for the early detection of HBV reactivation [8, 23].
In contrast to the above findings, several cases of HBV reactivation with viremia and emergence of HBsAg have been reported in patients with past HBV infection during immunosuppressive therapy for Crohn’s disease, ankylosing spondylitis, and RA [24–26]. Most recently, Urata et al. [21] have reported a high prevalence of HBV reactivation with marked increases in viral load in Japanese patients with RA and resolved HBV infection. In their study, 7 out of 135 patients (5.2%) in the resolved infection group became positive for HBV DNA (equal to or higher than 3.64 log copies/ml) at some point during a 12-month period of therapy. Notably, 52 patients with resolved HBV infection had been treated with biological agents; among these, 6 (11.5%) had HBV reactivation with an increased titer of viral DNA. Two patients showed particularly high levels of viremia (5 and 7.4 log copies/ml, respectively). The data on these 52 patients treated with biological agents suggest that RA patients with resolved HBV infection may be at a greater risk of reactivation of HBV when biological rather than nonbiological DMARDs are used for RA therapy. In the present study, however, no such tendency was evident when patients receiving anti-TNFα therapy and anti-TNFα-naïve patients were compared.
Kim et al. [17] have reported that, during anti-TNFα therapy for rheumatic diseases (median duration 25–27 months), clinically significant and persistent rises in serum levels of aminotransferase were observed at higher rates in a patient group with potential occult HBV infection (n = 88) than in the anti-HBc-negative group (n = 170), although viral load in these patients was not determined. Fourteen patients (15.9%) in the former group showed abnormal liver function, defined as a serum aminotransferase level at least twice the upper limit of normal. Using multiple logistic regression analysis, Kim and colleagues indicated that the presence of past infection was a significant risk factor associated with abnormal liver function, suggesting that serum aminotransferase may be used as a surrogate marker of possible reactivation of occult infection. In the present study, however, only one patient with past HBV infection presented with similar abnormal liver function; there was no difference in the rate of patients presenting with abnormal levels of serum aminotransferase between the anti-HBc-positive and -negative groups. In addition, the patients showing detectable levels of serum HBV-DNA had maintained serum ALT and AST levels within the normal ranges.
Prophylactic antiviral therapy has been recommended for HBsAg-positive patients receiving anti-TNFα agents, because such individuals are considered to be at high risk for viral reactivation [27–30]. At our institute, the prophylactic use of entecavir is indicated for HBsAg-positive patients who are scheduled for DMARD therapy. If RA is inadequately controlled despite treatment for at least 3 months with standard doses of conventional DMARDs, we consider biological DMARD therapy under close observation of HBV-DNA titers and ALT/AST levels. Entecavir has demonstrably potent anti-HBV activity, and long-term follow-up studies have shown that it confers sustained suppression of viral replication, yet has a low incidence of drug resistance and good tolerability, making it an ideal first-line agent for prolonged treatment of HBsAg carriers [31–33]. In the present study, 2 inactive HBsAg carriers had been started on anti-HBV prophylaxis with entecavir prior to the commencement of DMARD therapy and no reactivation of viral replication was observed. Likewise, Tamori et al. [22] have reported that among 5 patients positive for HBsAg, 3 pretreated with entecavir continued to receive MTX or etanercept without hepatic flares, whereas HBV reactivation occurred in the remaining 2 patients who had not received anti-HBV prophylaxis. The question is whether concomitant treatment with entecavir may be necessary for patients with past HBV infection and RA requiring an extended course of DMARD therapy including biological agents. HBV infection is one of the most common viral infections in humans, and Japan is one of its endemic areas: another group has reported that the prevalence of resolved HBV infection is 31.5% among Japanese RA patients [21], and 25% of the RA patients enrolled in the present study exhibited a serological pattern indicating past HBV infection. In addition, it is known that more than 20% of occult HBV carriers are negative for all HBV serological markers [16]. In the present study, the incidence of reactivation of HBV replication among this patient population was low even without antiviral prophylaxis. Although it cannot be denied that HBV reactivation occasionally leads to life-threatening liver failure [34], anti-HBV prophylaxis with entecavir is very expensive to maintain throughout long courses of therapy for RA; given the low incidence of HBV reactivation, anti-HBV prophylaxis may not be cost-effective. It therefore seems impractical to give prophylactic agents to all RA patients with past HBV infection who are to receive biological and nonbiological DMARDs.
The major findings of the present study are that HBsAg-negative/anti-HBc-positive serology was observed in one-fourth of RA patients and that the use of biological and nonbiological DMARDs is relatively safe in most RA patients with past HBV infection. Considering the inherently long-term nature of anti-RA therapy and the high costs of anti-HBV prophylaxis, universal prophylaxis for patients with RA and past HBV infection seems impractical. Careful monitoring of serum viral load seems to be the most rational approach to managing RA patients with past HBV infection who require anti-RA therapy.
References
Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84.
Smolen JS, Landewe R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–75.
Haroon N, Inman RD. Infectious complications of biological therapy. Curr Opin Rheumatol. 2009;21:397–403.
Saketkoo LA, Espinoza LR. Impact of biologic agents on infectious diseases. Infect Dis Clin N Am. 2006;20:931–61, viii.
Mori S, Cho I, Sugimoto M. A cluster of Pneumocystis jirovecii infection among outpatients with rheumatoid arthritis. J Rheumatol. 2010;37:1547–8.
Vassilopoulos D, Calabrese LH. Risks of immunosuppressive therapies including biologic agents in patients with rheumatic diseases and co-existing chronic viral infections. Curr Opin Rheumatol. 2007;19:619–25.
Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49:S156–65.
Calabrese LH, Zein NN, Vassilopoulos D. Hepatitis B virus (HBV) reactivation with immunosuppressive therapy in rheumatic diseases: assessment and preventive strategies. Ann Rheum Dis. 2006;65:983–9.
McMahon BJ. Recent advances in managing hepatitis B. F1000 Med Rep. 2010;2:11.
Marzano A, Angelucci E, Andreone P, Brunetto M, Bruno R, Burra P, et al. Prophylaxis and treatment of hepatitis B in immunocompromised patients. Dig Liver Dis. 2007;39:397–408.
Lubel JS, Testro AG, Angus PW. Hepatitis B virus reactivation following immunosuppressive therapy: guidelines for prevention and management. Intern Med J. 2007;37:705–12.
Stine JG, Khokhar OS, Charalambopoulos J, Shanmugam VK, Lewis JH. Rheumatologists’ awareness of and screening practices for hepatitis B virus infection prior to initiating immunomodulatory therapy. Arthritis Care Res (Hoboken). 2010;62:704–11.
Yazdany J, Calabrese L. Preventing hepatitis B reactivation in immunosuppressed patients: is it time to revisit the guidelines? Arthritis Care Res (Hoboken). 2010;62:585–9.
Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol. 2007;46:160–70.
Schmeltzer P, Sherman KE. Occult hepatitis B: clinical implications and treatment decisions. Dig Dis Sci. 2010;55:3328–35.
Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2:479–86.
Kim YJ, Bae SC, Sung YK, Kim TH, Jun JB, Yoo DH, et al. Possible reactivation of potential hepatitis B virus occult infection by tumor necrosis factor-alpha blocker in the treatment of rheumatic diseases. J Rheumatol. 2010;37:346–50.
Caporali R, Bobbio-Pallavicini F, Atzeni F, Sakellariou G, Caprioli M, Montecucco C, et al. Safety of tumor necrosis factor alpha blockers in hepatitis B virus occult carriers (hepatitis B surface antigen negative/anti-hepatitis B core antigen positive) with rheumatic diseases. Arthritis Care Res (Hoboken). 2010;62:749–54.
Charpin C, Guis S, Colson P, Borentain P, Mattei JP, Alcaraz P, et al. Safety of TNF-blocking agents in rheumatic patients with serology suggesting past hepatitis B state: results from a cohort of 21 patients. Arthritis Res Ther. 2009;11:R179.
Vassilopoulos D, Apostolopoulou A, Hadziyannis E, Papatheodoridis GV, Manolakopoulos S, Koskinas J, et al. Long-term safety of anti-TNF treatment in patients with rheumatic diseases and chronic or resolved hepatitis B virus infection. Ann Rheum Dis. 2010;69:1352–5.
Urata Y, Uesato R, Tanaka D, Kowatari K, Nitobe T, Nakamura Y, et al. Prevalence of reactivation of hepatitis B virus replication in rheumatoid arthritis patients. Mod Rheumatol. 2011;21:16–23.
Tamori A, Koike T, Goto H, Wakitani S, Tada M, Morikawa H et al. Prospective study of reactivation of hepatitis B virus in patients with rheumatoid arthritis who received immunosuppressive therapy: evaluation of both HBsAg-positive and HBsAg-negative cohorts. J Gastroenterol. (in press).
Raftery G, Griffiths B, Kay L, Kane D. Chronic viral hepatitis and TNF-alpha blockade. Rheumatology (Oxford). 2007;46:1381 (author reply 1381-2).
Gwak GY, Koh KC, Kim HY. Fatal hepatic failure associated with hepatitis B virus reactivation in a hepatitis B surface antigen-negative patient with rheumatoid arthritis receiving low dose methotrexate. Clin Exp Rheumatol. 2007;25:888–9.
Madonia S, Orlando A, Scimeca D, Olivo M, Rossi F, Cottone M. Occult hepatitis B and infliximab-induced HBV reactivation. Inflamm Bowel Dis. 2007;13:508–9.
Montiel PM, Solis JA, Chirinos JA, a Casis B, Sanchez F, Rodriguez S. Hepatitis B virus reactivation during therapy with etanercept in an HBsAg-negative and anti-HBs-positive patient. Liver Int. 2008;28:718–20.
Wendling D, Di Martino V, Prati C, Toussirot E, Herbein G. Spondyloarthropathy and chronic B hepatitis Effect of anti-TNF therapy. Joint Bone Spine. 2009;76:308–11.
Zingarelli S, Frassi M, Bazzani C, Scarsi M, Puoti M, Airo P. Use of tumor necrosis factor-alpha-blocking agents in hepatitis B virus-positive patients: reports of 3 cases and review of the literature. J Rheumatol. 2009;36:1188–94.
Carroll MB, Forgione MA. Use of tumor necrosis factor alpha inhibitors in hepatitis B surface antigen-positive patients: a literature review and potential mechanisms of action. Clin Rheumatol. 2010;29:1021–9.
Nathan DM, Angus PW, Gibson PR. Hepatitis B and C virus infections and anti-tumor necrosis factor-alpha therapy: guidelines for clinical approach. J Gastroenterol Hepatol. 2006;21:1366–71.
Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422–30.
Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–14.
Gonzalez SA, Keeffe EB. Entecavir for the long-term treatment of chronic hepatitis B. Expert Rev Anti Infect Ther. 2009;7:1053–62.
Umemura T, Tanaka E, Kiyosawa K, Kumada H. Mortality secondary to fulminant hepatic failure in patients with prior resolution of hepatitis B virus infection in Japan. Clin Infect Dis. 2008;47:e52–6.
Acknowledgments
This study was supported by research funds from the National Hospital Organization (NHO), Japan. I thank Dr. Shigetoshi Fugiyama of NTT West Japan Kyushu Hospital for his valuable advice on anti-HBV prophylaxis.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mori, S. Past hepatitis B virus infection in rheumatoid arthritis patients receiving biological and/or nonbiological disease-modifying antirheumatic drugs. Mod Rheumatol 21, 621–627 (2011). https://doi.org/10.1007/s10165-011-0458-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10165-011-0458-z