Abstract
Magnetic resonance imaging (MRI) is a useful tool for evaluating disease activity and therapeutic efficacy in rheumatoid arthritis (RA). However, conventional whole-body MRI is inconvenient on several levels. We have therefore developed a new low-field extremity MRI (compact MRI, cMRI) and examined its clinical utility. Thirteen RA patients treated with anti-tumor necrosis factor (TNF) biologics were included in the study. The MRI was performed twice using a 0.21-T extremity MRI system. The MRI images were scored using our proposed cMRI scoring system, which we devised with reference to the Outcome Measures in Rheumatology Clinical Trials RA MRI score (OMERACT RAMRIS). In our cMRI scoring system, synovitis, bone edema, and bone erosion are separately graded on a scale from 0 to 3 by imaging over the whole hand, including the proximal interphalangeal joint. The total cMRI score (cMRIS) is then obtained by calculating the total bone erosion score × 1.5 + total bone edema score × 1.25 + total synovitis score. In this study, one patient showed a progression of bone destruction even under low clinical activity, as assessed by the disease activity score on 28 joints (DAS28); however, another patient’s cMRIS decreased concurrently with the decrease in DAS28, with the positive correlation observed between ΔDAS28 and ΔcMRIS (R = 0.055, P < 0.05). We conclude that cMRI and cMRIS are useful for assessing total disease activity and as a method linking MRI image evaluation to clinical evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory autoimmune disease that predominantly affects the synovial membranes of joints. Persistent inflammation or synovitis leads to pannus formation and, ultimately, bone destruction. A therapeutic window [1] does exist early in the RA course; therefore, the development of better methods for the early diagnosis and treatment of RA is one of the prime objectives of rheumatologists. Conventional radiography is currently the major tool for diagnosing RA and monitoring the progression of joint destruction. However, because this technique visualizes only late signs of preceding disease activity, other diagnostic tools, such as magnetic resonance imaging (MRI), have been the focus of increasing attention in recent years. Magnetic resonance imaging is three- to sevenfold more sensitive than conventional radiography in terms of detecting joint erosion in early-stage RA [2, 3]. It can also detect synovitis, bone edema, and tenosynovitis that is not visible on conventional radiographic scans [4, 5]. Synovitis is among the earliest abnormalities observed in RA and is, in many cases, already apparent before a patient complains of joint pain or shows elevated serum C-reactive protein (CRP). The degree of bone marrow edema in metacarpalphalangeal (MCP) and wrist joints has recently been reported to be a more important predicator of radiographic progression in early RA than the degree of synovitis, erosion, or disease activity score based on 28 joints (DAS28) [6]. Evaluating bone edema by MRI may therefore assist clinicians in determining whether a patient should receive early and aggressive treatment to avoid subsequent joint damage.
While MRI may provide significant information about the course of RA not obtainable by conventional radiography, conventional whole-body, high-field MRI is more expensive in terms of both startup costs and maintenance fees, and it is not always convenient. In addition, claustrophobic patients and those suffering severe joint pain are sometimes unable to complete the examination. Low-field extremity MRI was recently developed to address these limitations; it is now commercially available and has been used clinically to evaluate RA. Low-field extremity MRI offers adequate performance at a lower cost and with greater comfort and convenience to the patient than conventional MRI [7, 8]. One strong disadvantage of this tool, however, is that the field of view (FOV) is too small to assess hand and wrist joints in one examination or in one sequence—and RA usually affects the wrist to proximal interphalangeal (PIP) joints. This is a major limiting factor in the success of low-field MRI for diagnosing of RA or assessing disease activity.
We have recently developed a new low-field extremity MRI system with a FOV large enough to simultaneously assess the entire wrist to PIP joint area. In the study reported here, we examined the clinical value of our low-field MRI system for assessing disease activity in RA patients treated with anti-tumor necrosis factor (TNF) biologics using the original scoring system.
Patients and methods
Patients and clinical assessments
Thirteen RA outpatients were enrolled in the study (two men and 11 women). The mean disease duration at evaluation was 6.2 years. Seven patients were treated with infliximab (IFX) and six with etanercept (ETN). Clinical disease activity was determined using the DAS28–CRP. Eleven patients had moderate or high disease activity before receiving anti-TNF biologics; the remaining patients had low clinical disease activity but showed bone destruction in the wrists, which had worsened significantly within the past year, as assessed by radiography. The IFX group also received an average methotrexate dose of 8 mg/week, with six patients also treated with prednisolone (average dose 7.1 mg/day). In the ETN group, four patients also received methotrexate (average dose 5 mg/week), and all six patients were taking prednisolone (average dose 5.3 mg/day). Table 1 presents additional sociodemographic data on these patients. All patients underwent two MRI assessments; the first was carried out at the time of starting the biologics (IFX group patients 5–7; ETN group patient 6) or within 7 and 9 months from the initial infusion of IFX and ETN (IFX group patients 1–4 and ETN group patients 1–5), respectively, and the second MRI assessment was within 8–16 months and 5–23 months from the first infusion for the respective groups.
New low-field extremity MRI system and MRI protocols
The new system is called compact MRI (cMRI). It comprises a permanent magnet, a gradient coil set, and an MRI console, generating a magnetic field strength of 0.21 T. The system occupies a total installation space of 4 m2. The magnet is placed in an electromagnetic shield room [1.6 (W) × 2.0 (H) × 2.4 (D)] to prevent external noise. Patients sit in front of the magnet and insert one hand into the radio frequency (RF) coil for MR imaging. Coronal three-dimensional (3-D) gradient recalled echo T1-weighted images [repetition time (TR)/echo time (TE) = 50/9 ms] were obtained with an image matrix size at 512 × 384 × 32, FOV of 20.48 × 15.36 × 6.4 cm, and a scan time of 7 min and 5 s. Coronal 3D fast short tau inversion recovery (STIR) images [TR/TE/inversion time (TI) = 1000/60/100 ms] were also obtained with an image matrix size of 256 × 256 × 8, FOV at 20.48 × 20.48 × 6.4 cm, and a scan time of 8 min and 30 s. Both hands were scanned in all patients. The total examination time, including patient positioning, required about 40 min.
Image evaluation and proposed compact MRI score
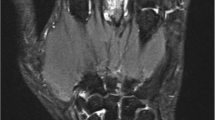
Magnetic resonance imaging findings are currently scored using the RA MRI scoring system (RAMRIS) as reported in the Outcome Measures in Rheumatology Clinical Trials (OMERACT) [9]. However, the RAMRIS system requires a two-dimensional (2-D) analysis, and our cMRI system can analyze only the coronary section; consequently, RAMRIS cannot be used in our system. Our modified MRI system can visualize joints from the wrist to PIP in only one image. We therefore evaluated the images using our original scoring system, to obtain a compact MRI score (cMRIS) referenced to RAMRIS. The cMRIS scores the degree of bone erosion, bone marrow edema, and synovitis in both hands. In this study, the MRI images were reviewed by one radiologist, who is a Board-certified radiologist (by the authority of the Japan Radiological Society), and by more than two rheumatologists. All patients’ information was blinded. Bone erosion and edema were defined using the OMERACT MRI joint pathology definition [10]. Bone erosion was defined by the presence of a sharply marginated bone region that was imaged as a loss of normal signal intensity of cortical bone and a loss of normal high signal characteristics, visible in two planes, with a cortical break seen in at least one plane on the T1-weighted image. Bone edema was defined a lesion within the trabecular bone, with ill-defined margins and signal characteristics consistent with increased water content that was imaged as high-intense signal on STIR and a low-intense signal on the T1-weighted image. Since we did not use gadolinium enhancement, synovitis was defined a high signal intensity on STIR that seemed anatomically to be the synovial area. The RAMRIS rates bone erosion from 0 to 10 by volume, while our scoring system rates bone erosion on a scale from 0 to 3 by volume. Bone edema and synovitis were scored on the same scale as RAMRIS. The PIP joints were scored by the same method used for the evaluating the MCP joints. This study evaluated 23 bones and 11 joints (Fig. 1). Bone erosion and edema were estimated in one to five MCP joints and one to five carpometacarpal (CM) joints, in two to five proximal and distal PIP joints, and in all wrist bones, except for the pisiforme, distal radius, and head of ulna. In the PIP and MCP joints, we evaluated each proximal and each distal side separately. and the score of the worse side was counted. Thus, the total estimation site of bone erosion and edema was 32. Synovitis, which was also scored on a scale from 0 to 3, was evaluated in two to five PIP joints, one to five MCP joints, and in the intercarpal and distal radioulnar joints. However, the intercarpal joint synovitis score was doubled because of its large volume. The overall score was calculated as follows: total synovitis score + 1.25 × total bone edema score + 1.5 × total bone erosion score [maximum total bone erosion score 207 (3 × 23 × 1.5 × 2), maximum total bone edema score 172.5 (3 × 23 × 1.25 × 2), maximum total synovitis score 72 {(3 × 10 + 3 × 1 × 2) × 2}; maximum cMRIS 451.5]. Further details of the scoring system are provided in Table 2.
Sites evaluated in calculating the compact magnetic resonance imaging (MRI) score. In this scoring system, 23 bones and 16 joints were evaluated. Pisiforme was excluded from the wrist bone evaluation. Bone erosion and edema were evaluated in 32 sites, and synovitis was evaluated in 11 sites. The score calculation is provided in detail in Table 2
Statistical analysis
The correlation between the changes in cMRIS (ΔcMRIS) and the DAS28–CRP (ΔDAS28) values was evaluated by Pearson’s correlation coefficient test. A value of P < 0.05 was considered to be significant.
Results
Evaluation of DAS28-CRP
The DAS28–CRP was evaluated prior to the biologics treatment and at the time of first and second MRI examinations. All patients of both treatment groups (IFX and ETN) except one showed a moderate-to-good response, as assessed by DAS28–CRP, and none showed a recurrence of disease activity (Fig. 2).
Serial changes in disease activity score on 28 joints (DAS28) and compact MRI score (cMRIS) between first (1st) and second (2nd) MRI examinations. All patients except one had a good or moderate response to the biologics, and none showed increased disease activity. The cMRIS scores generally decreased or remained constant. However, one patient of the infliximab group showed an increase in cMRIS score even under low disease activity (dotted line)
Changes in cMRI score
Figure 2 and Table 3 provide details on the MRI scores calculated from the first and second imaging examinations for all patients. The first imaging identified seven patients with synovitis and three with bone edema in the finger joints. All patients showed bone erosion in the first and second imaging. However, erosion of the finger joints did not worsen in any of the patients included in this study, with ten of 13 patients showing an improvement over the intervening time period. Synovitis was present in the wrist joints of 12 patients at the first imaging, and although persistent, the second imaging showed improved synovitis in the wrist joints in most patients. Ten of the 13 patients showed bone edema in the wrist joint at the first imaging; by the second imaging, seven of these patients showed improvement, and three patients showed deterioration. Patient 1 of the IFX group showed remarkable joint destructions over the treatment time, while the others remained the same or showed a slight improvement.
Relationship between cMRIS and DAS28-CRP
We evaluated the correlation between ΔcMRIS and ΔDAS28 in our small cohort and observed a positive correlation between the two scores (R = 0.055, P < 0.05; Fig. 3). However, one patient (IFX group patient 1) showed a very small change in the DAS28–CRP (2.66–2.83), indicating clinical remission but a marked worsening of the cMRIS (from 46.5 to 67.5).
Discussion
Rheumatoid arthritis is a chronic bone destruction disease that severely and progressively afflicts the patient’s daily activities. Biologics, including TNF blockers, have recently raised the hope of RA sufferers of dramatic improvements in joint mobility and prognosis. The Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes (TEMPO) study revealed the possibility of joint repair through treatment with ETN plus methotrexate [11]. However, many such studies used conventional radiography to evaluate bone erosion. Brown et al. [12] reported that about 96% patients treated with conventional disease-modifying antirheumatic drugs (DMARDs) achieved clinical remission according to the criteria of the American College of Rheumatology (ACR) and DAS28 score, but they still showed synovitis as assessed by MRI. The same percentage of asymptomatic patients with clinically normal joints also had synovitis based on MRI, while 46% showed bone marrow edema. In a comparative study on the therapeutic effectiveness of DMARDs and anti-TNF biologics, Martinez-Martinez et al. [13] also reported considerable synovitis based on MRI scans. This was still the case even in patients declared to be in clinical remission based on the biologics. These authors also reported no significant correlation between the improvement of clinical or laboratory data and MRI findings. Takes together, these studies stress the necessity of including an MRI examination in order to comprehensively evaluate total disease activity and joint damage in RA.
The imaging position and time needed for whole-body MRI makes it impractical for many rheumatologists to use and burdensome for the patient. Low-field extremity MRI is thus a valuable alternative that has recently become commercially available and has been tested for the diagnosis and monitoring of RA. Low-field extremity MRI improves patient comfort, is cost-effective for the institute, and yields equivalent results to whole-body MRI in terms of RA evaluation [14]. In support of this, using low-field extremity MRI, Savnik et al. [15] achieved a diagnostic accuracy for synovitis, bone edema, and bone erosion in RA comparable to that of high-field MRI.
Crues et al. [16] further reported that low-field dedicated-extremity MRI is more sensitive for detecting erosive changes in RA than radiography. In patients followed over 8 months, 30% demonstrated an increase in the size or number of erosions by MRI, while radiography revealed changes in only 0.8% of the patient cohort. Low-field dedicated-extremity MRI retains adequate imaging performance, but at a lower cost and with greater comfort and convenience for the patient. However, a limitation of low-field MRI is that the FOV is too small to enable an assessment of the hands and wrists in one examination. As wrist to PIP joints are usually affected in RA, examining the wrist to hand in one sequence is important for diagnosing and monitoring RA. Another disadvantage is that low-field MRI systems are not practical for small clinics to install because of their size and weight. To address these limitations and to render MRI more useful for RA diagnosis and treatment, we have developed a new low-field extremity MRI. This system has a large enough FOV to assess wrist to PIP joints in one examination, is lighter than its predecessor, and requires less total area to install.
The general adoption of MRI in general practice has also been hindered by a second problem. Many studies have used the RAMRIS OMERACT scoring method for MRI evaluation. However, this scoring method is too complex for use in daily medical examinations and treatments. We have used a new scoring system, cMRIS, for evaluating disease activity in RA patients treated with anti-TNF biologics. This method evaluates bone erosion, bone edema, and synovitis as well as RAMRIS. The RAMRIS scored bone erosion on a scale of 0–10 by its volume, which may be inconvenient. In addition, the RAMRIS method requires 2-D analysis. Therefore, we have improved this point and developed cMRIS. The cMRIS scores bone erosion on a scale of 0–3 by its volume, just as the method used for edema and synovitis. Considering the irreversibility of each finding, we decided that the coefficients for each finding should be 1.5 for bone erosion, 1.25 for bone edema, and 1 for synovitis. Based on the positive correlation that we obtained between ΔDAS28 and ΔcMRIS, we consider our scoring system to be useful in linking MRI image evaluation to clinical evaluation. However, a future large-scale study will be necessary to examine whether these coefficients are appropriate. Another problem is that synovitis cannot be evaluated precisely because we did not use gadolinium enhancement in our MRI system. As gadolinium enhancement requires intravenous injection and may induce severe side effects, such as nephrogenic systemic fibrosis, it cannot always be used in daily practice, especially in a small clinic. The problem of inaccuracy due to not using gadolinium can be solved to some extent through the acquisition of experience. As the aim of our study was to establish the evaluation of RA disease activity by MRI in daily practice, we did not use gadolinium enhancement and instead developed an easier system to image and to facilitate the evaluations of these images.
In almost all patients, a positive correlation was observed between ΔDAS28 and ΔcMRIS. However, one patient in the IFX group showed a worsening of bone destruction when evaluated by cMRI even though the estimated DAS28–CRP indicated clinical remission. Prior to treatment, this patient had moderate disease activity (DAS28–CRP 4.1). She then responded well to the treatment and remained close to clinical remission during the study. The MRI scan showed a DAS28–CRP of 2.66 at the first imaging and 2.83 at the second imaging. However, both bone edema and erosion had worsened, as evidenced by the MRI scan. This patients provides good proof of how we can understand real disease activity using not only the DAS28 but also the cMRI in daily practice. In the future, rheumatologists should estimate real disease activity by MRI and other tools in addition to clinical activity as estimated by the DAS28.
Low-field extremity MRI has been reported to record a lower sensitivity than whole-body MRI in terms of bone edema assessment [17], and different sensitivities have been reported among different models [18]. We did not compare our cMRI image and the 1.5-T whole-body MRI image. However, work is now ongoing to develop an improved system, the 0.3-T MRI machine, called the compacTscan, which will enable a higher resolution and sensitivity imaging, and a more precise diagnosis of RA. To date, we have compared the 0.3-T cMRI image and the 1.5-T whole-body MRI image in three patients and obtained almost the same results (data not shown). The low-field extremity MRI is convenient for both patients and rheumatologists, and its use in daily practice could assist clinicians both in making an earlier diagnosis of RA and a more precise estimation of disease activity. The hope is that joint prognosis of RA patients will be improved using cMRI.
In conclusion, the results of our study have shown a positive correlation between ΔcMRIS and ΔDAS28, suggesting that cMRI and the cMRIS are useful for estimating total disease activity and joint damage in RA.
References
Cush JJ. Early rheumatoid arthritis—is there a window of opportunity? J Rheumatol Suppl. 2007;80:1–7.
Klarlund M, Ostergaard M, Jensen KE, Madsen JL, Skjødt H, Lorenzen I. Magnetic resonance imaging, radiography, and scintigraphy of the finger joints: one year follow up of patients with early arthritis The TIRA Group. Ann Rheum Dis. 2000;59:521–8.
McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PL, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis. 1999;58:156–63.
Taylor PC. The value of sensitive imaging modalities in rheumatoid arthritis. Arthritis Res Ther. 2003;5:210–3.
Ostergaard M, Szkudlarek M. Imaging in rheumatoid arthritis—why MRI and ultrasonography can no longer be ignored. Scand J Rheumatol. 2003;32:63–73.
Haavardsholm EA, Bøyesen P, Østergaard M, Schildvold A, Kvien TK. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow edema predicts erosive progression. Ann Rheum Dis. 2008;67:794–800.
Taouli B, Zaim S, Peterfy CG, Lynch JA, Stork A, Guermazi A, et al. Rheumatoid arthritis of the hand and wrist: comparison of three imaging techniques. AJR Am J Roentgenol 2004;182:937–43.
Savnik A, Malmskov H, Thomsen HS, Bretlau T, Graff LB, Nielsen H, et al. MRI of the arthritic small joints: comparison of extremity MRI (0.2 T) vs high-field MRI (1.5 T). Eur Radiol. 2001;11:1030–8.
Østergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–6.
McQueen F, Lassere M, Edmonds J, Conaghan P, Peterfy C, Bird P, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Summary of OMERACT 6 MR Imaging Module. J Rheumatol. 2003;30:1387–92.
Klareskog L, van der Heijde D, Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blinded randomized controlled trial. Lancet. 2004;363:675–81.
Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54:3761–73.
Martinez-Martinez MU, Cuevas-Orta E, Reyes-Vaca G, Baranda L, Gonzalez-Amaro R, Abud-Mendoza C. Magnetic resonance imaging in patients with rheumatoid arthritis with complete remission treated with disease-modifying antirheumatic drugs or anti-tumour necrosis factor alpha agents. Ann Rheum Dis. 2007;66:134–5.
Taouli B, Zaim S, Peterfy CG, Lynch JA, Stork A, Guermazi A, et al. Rheumatoid arthritis of the hand and wrist: comparison of three imaging techniques. AJR Am J Roentgenol.. 2004;182:937–43.
Savnik A, Malmskov H, Thomsen HS, Bretlau T, Graff LB, Nielsen H, et al. MRI of the arthritic small joints: comparison of extremity MRI (0.2 T) vs high-field MRI (1.5 T). Eur Radiol. 2001;11:1030–8.
Crues JV, Shellock FG, Dardashti S, James TW, Troum OM. Identification of wrist and metacarpophalangeal joint erosions using a portable magnetic resonance imaging system compared to conventional radiographs. J Rheumatol. 2004;31:676–85.
Bird P, Ejbjerg B, Lassere M, Østergaard M, McQueen F, Peterfy C, et al. A multireader reliability study comparing conventional high-field magnetic resonance imaging with extremity low-field MRI in rheumatoid arthritis. J Rheumatol. 2007;34:854–6.
Duer-Jensen A, Ejbjerg B, Albrecht-Beste E, Vestergaard A, Møller Døhn U, Lund Hetland M, et al. Does low-field dedicated extremity MRI (E-MRI) reliably detect RA bone erosions? A comparison of two different E-MRI units and conventional radiography with high resolution CT. Ann Rheum Dis. 2008. doi: 10.1136/ard.2008.093591.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Suzuki, T., Ito, S., Handa, S. et al. A new low-field extremity magnetic resonance imaging and proposed compact MRI score: evaluation of anti-tumor necrosis factor biologics on rheumatoid arthritis. Mod Rheumatol 19, 358–365 (2009). https://doi.org/10.1007/s10165-009-0172-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10165-009-0172-2