Abstract
The effects of rebamipide on dry mouth and salivary secretion in Sjögren’s syndrome patients were investigated in a double-blind placebo-controlled study. Rebamipide (100 mg TID) or placebo was administered for eight weeks and patient-assessed improvement of dry mouth and increase in salivary secretion measured by the Saxon test were evaluated. At two, four, and eight weeks, dry mouth improvement rates were, respectively, 26.0, 44.0, and 46.9% for rebamipide and 20.0, 27.1, and 39.1% for placebo, and mean increases in salivary secretion were, respectively, 0.14, 0.24, and 0.35 g for rebamipide and 0.03, 0.09, and 0.17 g for placebo, indicating higher values in the rebamipide group for both parameters at all timepoints but no significant differences between the two groups. Analysis by baseline characteristics suggested a statistically significant salivary secretion increasing effect of rebamipide in cases of primary Sjögren’s syndrome. No difference in the incidence of adverse events was seen between the two groups, confirming the safety of rebamipide. As a salivary secretion increasing effect was strongly suggested in cases of primary Sjögren’s syndrome, further study on the administration of rebamipide for the treatment of dry mouth in patients with Sjögren’s syndrome is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sjögren’s syndrome is an autoimmune disease involving chronic inflammation of exocrine glands such as the salivary and lacrimal glands and in some cases also systemic organ lesions, and its etiology is not yet known. The prevalence of Sjögren’s syndrome is estimated to be between 0.5% and 1.5%, and approximately 90% of patients are women. Sjögren’s syndrome occurring in association with connective tissue diseases such as rheumatoid arthritis or systemic lupus erythematosus is referred to as secondary Sjögren’s syndrome, and occurrence without these diseases is classified as primary Sjögren’s syndrome. The major symptoms of Sjögren’s syndrome are dry mouth and dry eye, and the primary cause is considered to be impaired function of the salivary and lacrimal glands resulting from damage to acini by periductal infiltration of lymphocytes [1]. Persistent dryness of the mouth not only causes oral pain and discomfort, but also markedly decreases patient quality of life due to such problems as sleep disturbance, difficulties with eating, and difficulties with speaking [2].
Currently the first-choice treatment for dry mouth in Sjögren’s syndrome is sialogogic drugs such as cevimeline hydrochloride and pilocarpine hydrochloride, which stimulate the sialogogic M3 muscarinic receptors on the cell surfaces of exocrine glands [3, 4]. However, as these drugs only provide symptomatic therapy, there is a strong need for the development of a therapeutic method that will directly address the pathophysiology of Sjögren’s syndrome. In recent years, attention has focused on treatment using anti-CD20 antibody [5], while anti-BAFF therapy [6] and the immunosuppressive agent mizoribine [7, 8] have also shown some efficacy, but none of these has entered practical use.
Rebamipide (Mucosta®) has been in clinical use in Japan and various other Asian countries since 1990 as a drug for the treatment of gastritis and gastric ulcer. Rebamipide has a mucosa-protective effect based on its promotion of endogenous prostaglandin biosynthesis and other actions, and recent studies have also shown it to have various inflammation inhibitory effects, including inhibition of neutrophilic leukocyte activation and suppression of inflammatory cytokine production by monocytes and gastric mucosal cells [9, 10]. In a preclinical study, saliva volume was increased following oral administration of rebamipide in an NFS/sld mouse model of Sjögren’s syndrome, and histopathological examination revealed inhibition of TUNEL-positive apoptosis of the salivary and lacrimal gland excretory duct cells and suppression of CD4+ T-cell activation and Th1 cytokine (IL-2 and IFN-γ) production [11]. In a clinical study, Oka et al. reported that when rebamipide was administered orally at 300 mg/day for eight weeks to 21 patients with Sjögren’s syndrome, subjective symptoms related to dry mouth were significantly improved in comparison with the pretreatment baseline, and a significant increase in salivary secretion versus the baseline was also observed [12]. Based on the results of these preclinical and clinical studies, we conducted a double-blind placebo-controlled comparative study to investigate rebamipide for the treatment of dry mouth symptoms in patients with Sjögren’s syndrome.
Materials and methods
This study was conducted as a prospective, double-blind, placebo-controlled, multicenter trial.
Study subjects
Inclusion criteria
Sjögren’s syndrome patients with dry mouth symptoms who met all of the following inclusion criteria were eligible for enrollment in this study:
-
(1)
Age was 20 years or older at the time that informed consent was obtained
-
(2)
Severity of dry mouth symptoms was “mild” or greater at both the start and end of the run-in observation period, with any difference between the start and end of the run-in observation period limited to one grade or less
-
(3)
Difference in the Saxon test results between the start and end of the run-in observation period was 1 g or less and a result of 3 g or less was obtained for the Saxon test performed at the end of the run-in observation period
Exclusion criteria
Patients to whom any of the following criteria applied were excluded from participation in this study:
-
(1)
Patients with dry mouth symptoms that were clearly attributable to a cause other than Sjögren’s syndrome
-
(2)
Patients in whom the Saxon test could not be performed (patients with dentures, post crowns, etc.)
-
(3)
Patients who had received rebamipide within three months prior to obtaining informed consent
-
(4)
Female patients who were pregnant, possibly pregnant, or lactating, or who desired to become pregnant
-
(5)
Patients with hypersensitivity to rebamipide
-
(6)
Patients who had received another investigational drug within three months prior to obtaining informed consent
Study design and schedule
A run-in observation period of 2–4 weeks was set for patients from whom informed consent to participate in the study was obtained. Only those patients who satisfied all of the inclusion and exclusion criteria described above based on the examinations performed at the start and end of the run-in observation period were allocated at a ratio of 1:1 to the rebamipide and placebo groups and allowed to advance to the administration period. The dose and dosing regimen for rebamipide were 100 mg administered orally three times a day (100 mg TID) for eight weeks. After the start of administration, subjects were required to visit the study site at two, four, and eight weeks, and the examinations specified below were performed to evaluate efficacy and safety.
Throughout the study period, cevimeline hydrochloride, anethole trithione, and other treatments administered to improve dry mouth symptoms were prohibited. Although there were no general restrictions on the use of locally applied formulations (synthetic saliva and various oral care products) for temporary alleviation of dry mouth symptoms, their use was prohibited prior to examination on the specified examination days. There were no restrictions regarding drugs that had been used since before the start of the study for the treatment of underlying diseases and complications, but the doses and dosing regimens of such drugs had to remain unchanged during the study period.
The rebamipide tablets and placebo tablets used in this study were indistinguishable in appearance. With the exception that the placebo tablets contained no rebamipide, the composition of the placebo tablets was the same as that of the rebamipide tablets.
Overall improvement in dry mouth symptoms
During visits to the study site at the start and end of the run-in observation period, subjects self-assessed the overall severity of their dry mouth symptoms on the following four-grade scale:
-
(1)
None (almost no discomfort or inconvenience due to dryness of the mouth)
-
(2)
Mild (occasional discomfort or inconvenience due to dryness of the mouth; almost no impediment to daily living)
-
(3)
Moderate (discomfort or inconvenience due to dryness of the mouth felt on a daily basis; some impediment to daily living)
-
(4)
Severe (discomfort or inconvenience due to dryness of the mouth strongly felt on a daily basis; considerable impediment to daily living)
At visits to the study site at two, four, and eight weeks after the start of administration, subjects self-assessed the overall change in their dry mouth symptoms in comparison with their symptoms before the start of treatment on the following four-grade scale:
-
(1)
Markedly improved (clearly better)
-
(2)
Improved (better)
-
(3)
Unchanged (almost no difference)
-
(4)
Aggravated (worse)
The improvement rate was calculated by defining improvement as an assessment of either (1) markedly improved or (2) improved.
Volume of salivary secretion
During visits of the subject to the study site at the start and end of the run-in observation period, and at two, four, and eight weeks after the start of administration, the Saxon test was performed according to the method reported by Kohlen and Winter [13], and the volume increase and the rate of increase in salivary secretion were calculated in reference to the value obtained at the start of administration.
Various symptoms accompanying dry mouth
At visits to the study site at the start of administration and at two, four, and eight weeks, subjects self-assessed the severity of their various symptoms accompanying dry mouth using a 100-mm visual analog scale (VAS). The following six items were assessed as symptoms: feeling of dryness in the mouth (inside of the mouth feels parched), saliva abnormality (inside of the mouth feels sticky), thirst (desire to drink water because mouth feels dry), difficulty eating (dry foods such as crackers and bread become stuck in the mouth or throat), difficulty speaking (difficult to continue speaking without drinking water), and sleep disturbance (awakening during sleep due to dryness of the mouth). The change (∆ mm) for each item was calculated in comparison with the value at the start of administration.
Objective findings of dry mouth
At subject visits to the study site at the start of administration and at two, four, and eight weeks, based on the findings of the investigator’s examination, the four items of dryness of the lingual surface, dryness of the oral mucosa, angular stomatitis, and decrease in sublingual saliva retention were each assessed on the following four-grade scale:
-
(1)
None
-
(2)
Mild
-
(3)
Moderate
-
(4)
Severe
Safety
At visits to the study site at the start of administration and at two, four, and eight weeks, subjects were questioned regarding any abnormal findings observed since the previous visit. Laboratory tests (hematology, biochemistry, and urinalysis) were performed at the start of administration and at four and eight weeks. Any abnormal findings observed in either subject interviews or laboratory tests were defined as adverse events, and any adverse event for which a causal relationship of the study drug could not be denied was handled as an adverse drug reaction.
Size of study population
The improvement rate in the primary assessment parameter was expected to be 30% for the placebo group and 60% for the rebamipide group. Based on those estimates, the number of subjects necessary to detect a significant difference between the two groups by chi-square test (two-sided significance level of 5% and power of 80%) was calculated to be 42 per group. Considering the possibility of some withdrawals, the size of the study population was set at 50 subjects per group for a total of 100 subjects.
Statistical analysis
All analyses were performed using SAS Ver. 8.2 (SAS Institute Japan, Ltd., Tokyo, Japan). Comparisons between the two groups were made by Wilcoxon signed rank sum test or chi-square test for baseline characteristics, by chi-square test for overall improvement rates in dry mouth symptoms, and by t-test for volume of salivary secretion. As this was an exploratory study, P values were not adjusted for multiplicity. Results for 95% confidence intervals are displayed as two-sided 95% confidence intervals calculated by normal approximation.
Results
This study was conducted between April 2005 and January 2006 through the cooperation of 12 medical institutions located throughout Japan. The institutional review board of each medical institution approved the study beforehand. Written informed consent was obtained from each study subject prior to the start of the run-in observation period.
Subjects included in the analysis and their baseline characteristics
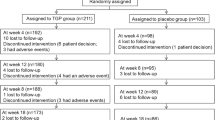
Informed consent to participate in the study was obtained from 128 subjects. During the run-in observation period, 24 subjects were withdrawn due to withdrawal of consent, failure to satisfy the inclusion or exclusion criteria, or deviation of the study protocol, and the remaining 104 subjects were randomized to the two groups (53 to the rebamipide group and 51 to the placebo group). Of the randomized subjects, 50 in the rebamipide group and 46 in the placebo group completed the eight weeks of treatment. Reasons for withdrawal during the administration period were one case of discontinuation at the subject’s request, one case of discontinuation due to an adverse event and one case of discontinuation due to aggravation of an underlying complication in the rebamipide group, and one case of discontinuation at the subject’s request, two cases of discontinuation due to an adverse event and two cases of discontinuation due to insufficient efficacy in the placebo group (see Fig. 1). Of the 104 randomized subjects, excluding one case of protocol deviation in the rebamipide group, and two cases in the rebamipide group and one case in the placebo group with missing data on the primary assessment parameter, 50 subjects in the rebamipide group and 50 subjects in the placebo group were included in the per protocol set (PPS) analysis population. The efficacy analyses of rebamipide were performed for the PPS.
The baseline characteristics of the subjects in the PPS are shown in Table 1. No differences were seen between the two groups in gender, age, Sjögren’s syndrome diagnosis (primary or secondary), duration of Sjögren’s syndrome, type of connective tissue disease, or history of cevimeline hydrochloride use. Nor were any differences observed between the two groups regarding the severity of dry mouth symptoms or volume of salivary secretion at the start of administration.
Overall improvement in dry mouth symptoms
Overall improvement rates for dry mouth symptoms are shown in Table 2. Improvement rates (rebamipide group vs. placebo group) were 26.0% vs. 20.0% (P = 0.48) at two weeks, 44.0% vs. 27.1% (P = 0.08) at four weeks, and 46.9% vs. 39.1% (P = 0.44) at eight weeks. Higher improvement rates were seen for the rebamipide group at all timepoints, with the greatest difference seen after four weeks of treatment. However, no statistically significant differences were seen between the two groups.
Volume of salivary secretion
Increases in salivary secretion volume and the rates of increase in comparison with the baseline values obtained before the start of administration are shown in Fig. 2. Group mean increases in salivary secretion volume (g) (rebamipide group vs. placebo group) were 0.14 ± 0.40 vs. 0.03 ± 0.47 (P = 0.21) at two weeks, 0.24 ± 0.46 vs. 0.09 ± 0.52 (P = 0.14) at four weeks, and 0.35 ± 0.54 vs. 0.17 ± 0.58 (P = 0.11) at eight weeks. Mean increase rates (ratio vs. value of 1 for the baseline) (rebamipide group vs. placebo group) were 1.16 ± 0.38 vs. 1.12 ± 0.56 (P = 0.68) at two weeks, 1.28 ± 0.53 vs. 1.19 ± 0.64 (P = 0.48) at four weeks, and 1.43 ± 0.56 vs. 1.22 ± 0.62 (P = 0.08) at eight weeks. Although no statistically significant differences between the two groups were seen in either the volume increase or rate of increase, the rebamipide group showed a tendency towards an increase in salivary secretion with continuation of treatment.
Changes in salivary secretion (PPS). For the Saxon tests performed at two, four, and eight weeks after the start of administration, the volume increase (value at each measurement point − baseline value) (g) and the rate of increase (value at each measurement point/baseline value) in salivary secretion were calculated for each subject using the value obtained at the start of administration as the baseline value, and the group mean ± SD values were determined. The significance of the difference between groups was determined by t-test
Various symptoms accompanying dry mouth
Mean changes (decreasing values corresponding to an improvement in symptoms) from the start of administration in a 100-mm VAS for the six items of feeling of dryness in the mouth, saliva abnormality, thirst, difficulty eating, difficulty speaking, and sleep disturbance are shown in Fig. 3. Of the six items, feeling of dryness in the mouth and difficulty speaking showed a tendency to improve with continuation of treatment in the rebamipide group in comparison with the placebo group, although no statistically significant differences were seen between the two groups. Saliva abnormality (the inside of the mouth feels sticky) was the only item to show a tendency for a lack of improvement.
Mean change in VAS for each symptom accompanying dry mouth (PPS). The severities of six symptoms were self-assessed by subjects at the start of administration and at two, four, and eight weeks using a 100-mm VAS (left edge: none; right edge: very severe), and the length (mm) of the subject’s evaluation from the left edge was measured. Changes from the start of administration at two, four, and eight weeks were calculated for each subject, and the group mean ± SD values were determined. The significance of the difference between groups was determined by t-test
Objective findings of dry mouth
Changes in score (improvement of one grade for the four grades of none, mild, moderate, and severe tabulated as a change of −1) from the pretreatment baseline after eight weeks of administration are shown in Fig. 4 as the distribution of the number of subjects with each change in score. The rebamipide group showed a tendency for a larger number of subjects distributed toward greater improvement for all four items of dryness of the lingual surface, dryness of the oral mucosa, angular stomatitis, and decrease in sublingual saliva retention. However, the differences between the two groups were small and not statistically significant.
Changes in objective findings of dry mouth at eight weeks (PPS). At each subject visit to the study site, the investigator assessed the four items of dryness of the lingual surface, dryness of the oral mucosa, angular stomatitis, and decrease in sublingual saliva retention on a four-grade scale of (1) none, (2) mild, (3) moderate, and (4) severe. The change in score for each item at eight weeks from that before the start of administration was calculated, and the distribution of the number of subjects with each change in score was determined. Assessments that were graded (1) none at both timepoints were excluded from tabulation. The significance of the difference between groups was determined by Wilcoxon signed rank sum test
Safety
Safety was evaluated in all subjects allocated to either the rebamipide group or the placebo group. Adverse events were observed in 32 of 53 subjects (60.4%) in the rebamipide group and 34 of 51 subjects (66.7%) in the placebo group. The most frequently observed adverse events were gastrointestinal disorders in both groups. Withdrawal from the study due to an adverse event occurred in one subject (blood creatinine increased) in the rebamipide group and two subjects (pneumonia and genital pruritus) in the placebo group. Adverse drug reactions (adverse events for which a causal relationship of the study drug could not be denied) were observed in 11 of 53 subjects (20.8%) in the rebamipide group and 18 of 51 subjects (35.3%) in the placebo group. There were no significant differences between the rebamipide group and the placebo group in the incidences or types of adverse events and adverse drug reactions. Therefore, the preferable safety profile of rebamipide was confirmed.
Discussion
There is currently no therapeutic method that directly addresses the pathophysiology of Sjögren’s syndrome, and available therapy primarily consists of symptomatic treatment of dry mouth and dry eye. As an example of a symptomatic treatment, cyclosporine A ophthalmic solution has been approved in the United States for dry eye [14]. For the treatment of dry mouth, a low dose of interferon α by the oromucosal route has been reported to have a salivary secretion increasing effect and is expected to provide effective therapy [15], and as the mechanism of action, data suggesting upregulation of the gene expression of aquaporin 5 has also been reported [16]. Oral administration of hydroxychloroquine has been used as a systemic method of therapy in the United States, but while it is considered effective against some systemic symptoms such as arthralgia and rash, it does not have a potent effect on salivary secretion [17, 18]. Adrenal corticosteroid hormones are also occasionally used as a systemic therapy, and they are considered effective at increasing salivary secretion and in treating histological abnormalities [19, 20], but as the majority of Sjögren’s syndrome patients are middle-aged women, adverse drug reactions—including osteoporosis, diabetes, cardiovascular effects, periodontal disease, and oral candidiasis—are major issues, so that hormonal treatment is not generally recommended.
Muscarinic receptor agonists such as cevimeline hydrochloride and pilocarpine hydrochloride are used as the first choice treatment for dry mouth symptoms, but some patients do not show satisfactory improvement with these drugs. In addition, nausea, vomiting, excessive perspiration, and other adverse drug reactions due to the pharmacological action of muscarinic receptor agonists are often seen, and these drugs are also contraindicated in patients with serious heart disease, bronchial asthma, and other disorders. For these reasons there is a need for the development of a safer, more effective drug with a mechanism of action that is different from that of muscarinic receptor agonists. In recent years, attention has focused on using anti-CD20 antibody [5] as a therapy for Sjögren’s syndrome, while anti-BAFF therapy [6] and the immunosuppressive agent mizoribine [7, 8] have also been reported to show efficacy, but the establishment of an actual therapeutic method has not yet been realized.
Previous studies have demonstrated rebamipide to have various inflammation inhibitory effects, including suppression of inflammatory cytokine production by monocytes [21] and free radical scavenging activity [22]. Thus, as rebamipide possesses an anti-inflammatory action and various other effects in addition to its mucosa-protective activity, it is considered that it would also show efficacy against other disorders as well as gastrointestinal disorders. As one example, since rebamipide has been reported to increase the amount of mucin-like substances in the conjunctiva and cornea in an N-acetylcysteine-treated in vivo model [23], and also to increase the proliferation of cultured rat conjunctival goblet cells [24], the compound is currently being evaluated for the treatment of dry eye by topical ophthalmological administration.
Based on the above background, the present study was conducted to exploratively evaluate the efficacy of rebamipide in treating dry mouth symptoms in patients with Sjögren’s syndrome, which is characterized by inflammation of the salivary glands due to lymphocytic infiltration and impairment of the glands’ salivary secretion function. From the results of this study, the overall improvement in dry mouth symptoms, which was the primary assessment parameter, was higher in the rebamipide group than in the placebo group at all measurement timepoints, although the differences between the two groups were not statistically significant. Because the overall improvement of dry mouth symptoms is a subjective index that depends on the study subjects’ self-assessment of “a feeling of dryness” in comparison with a subjective memory of their condition prior to the start of treatment, it cannot be denied that with the passage of time the subjects’ memory of their pretreatment symptoms may dim and their criteria for judging the improvement of their subjective symptoms upon treatment may become quite vague. In comparison with subjective symptoms, salivary secretion volume is an objective index. The rebamipide group showed a tendency for increased salivary secretion with continuation of treatment, although the differences between the groups were not statistically significant. A crucial point from an exploratory point of view was that analysis by subject baseline characteristics showed a clear difference in the effect of rebamipide on salivary secretion between cases of primary and secondary Sjögren’s syndrome. While the rebamipide group showed a statistically significant increase in salivary secretion in comparison with the placebo group in cases of primary Sjögren’s syndrome, no increase in salivary secretion was seen in the rebamipide group for cases of secondary Sjögren’s syndrome (see Fig. 5). In analysis by other baseline characteristics, rebamipide showed a marked salivary secretion increasing effect in non-elderly subjects younger than 65 years (increase in salivary secretion volume at eight weeks: 0.42 ± 0.61 g in the rebamipide group vs. 0.17 ± 0.44 g in the placebo group; P = 0.07) and in subjects in whom the baseline Saxon test result was 2–3 g, indicating mildly decreased salivary secretion (increase in salivary secretion volume at eight weeks: 0.79 ± 0.79 g in the rebamipide group vs. 0.28 ± 0.34 g in the placebo group; P = 0.16).
Similar to salivary secretion, dry mouth symptoms also tended to show higher rates of improvement by rebamipide in cases of primary Sjögren’s syndrome, in non-elderly subjects, and in subjects with mildly decreased salivary secretion. In cases of primary Sjögren’s syndrome, the overall improvement rates for dry mouth symptoms (rebamipide group vs. placebo group) were 27.0% vs. 11.4% (P = 0.10) at two weeks, 48.6% vs. 23.5% (P = 0.03) at four weeks, and 48.6% vs. 37.5% (P = 0.35) at eight weeks, with a statistically significant difference seen at four weeks. There were no differences in baseline characteristics between the rebamipide and placebo groups for subjects with primary Sjögren’s syndrome (see Table 3).
The biggest difference between cases of primary and secondary Sjögren’s syndrome is the higher frequency of concomitantly administered corticosteroids, immunosuppressants, and anti-inflammatory agents for treatment of the various connective tissue disease complications accompanying secondary Sjögren’s syndrome. In this study, corticosteroids were concomitantly administered in 13 of 28 subjects (46%) with secondary Sjögren’s syndrome, versus only 8 of 72 subjects (11%) with primary Sjögren’s syndrome. Corticosteroids have been reported to have an improving effect on Sjögren’s syndrome [19, 20]. Further evidence suggesting that these concomitantly administered drugs may have influenced the results of the study was that in the placebo group, while almost no increase in salivary secretion was seen in subjects with primary Sjögren’s syndrome, a marked salivary secretion increasing effect was observed in subjects with secondary Sjögren’s syndrome.
In future studies, it is considered that the efficacy of rebamipide could be better clarified by selecting subjects that are more suitable for evaluation, such as by limiting enrollment to patients with primary Sjögren’s syndrome.
Kohashi et al. reported rebamipide’s mechanism of action for promoting salivary secretion in the NFS/sld mouse [11]. They found that the development of autoimmune sialoadenitis is prevented by rebamipide’s suppression of T-cell activation and inhibition of apoptosis of salivary and lacrimal gland excretory duct epithelial cells, suggesting that these actions may also promote increased salivary secretion. There are also data from unpublished animal studies suggesting that rebamipide has a direct salivary secretion stimulating action. Because rebamipide does not have an M3 muscarinic receptor stimulating action, further study is needed to clarify the compound’s mechanism of action for promoting salivary secretion.
Unlike sialogogic drugs, which are muscarinic receptor agonists, rebamipide’s effects on increasing salivary secretion and on dry mouth symptoms are not observed in a short period of time. Rebamipide will be an agent that is used to supplement sialogogic drugs rather than to replace sialogogic drugs, with the objective of protecting the oral mucosa and inhibiting secretory gland disorders via a mechanism of action that is different from that of a muscarinic receptor agonist. Further studies are necessary to demonstrate that potential by evaluating rebamipide’s long-term therapeutic effects, such as whether or not it actually improves salivary gland hypofunction and histological disorders.
References
Moutsopoulos HM. Sjögren’s syndrome: autoimmune epithelitis. Clin Immunol Immunopathol. 1994;72:162–5.
Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321–31.
Ramos-Casals M, Brito-Zerón P. New approaches in Sjögren’s syndrome therapy. Expert Rev Clin Immunol. 2007;3:195–204.
Thanou-Stavraki A, James JA. Primary Sjogren’s syndrome: current and prospective therapies. Semin Arthritis Rheum. 2008;37:273–92.
Devauchelle-Pensec V, Pennec Y, Morvan J, Pers JO, Daridon C, Jousse-Joulin S, et al. Improvement of Sjögren’s syndrome after two infusions of rituximab (anti-CD20). Arthritis Rheum. 2007;57:310–7.
Pers JO, Devauchelle V, Daridon C, Bendaoud B, Le Berre R, Bordron A, et al. BAFF-modulated repopulation of B lymphocytes in the blood and salivary glands of rituximab-treated patients with Sjögren’s syndrome. Arthritis Rheum. 2007;56:1464–77.
Nakayamada S, Saito K, Nakatsuka K, Nakano K, Tokunaga M, Sawamukai N, et al. Efficacy of mizoribine treatment in patients with Sjögren’s syndrome: an open pilot trial. Mod Rheumatol. 2003;13:339–45.
Nakayamada S, Saito K, Umehara H, Ogawa N, Sumida T, Ito S, et al. Efficacy and safety of mizoribine for the treatment of Sjögren’s syndrome: a multicenter open-label clinical trial. Mod Rheumatol. 2007;17:464–9.
Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A. Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig Dis Sci. 1998;43:5S–13S.
Arakawa T, Higuchi K, Fujiwara Y, Watanabe T, Tominaga K, Sasaki E, et al. 15th anniversary of rebamipide: looking ahead to the new mechanisms and new applications. Dig Dis Sci. 2005;50:S3–S11.
Kohashi M, Ishimaru N, Arakai R, Hayashi Y. Effective treatment with oral administration of rebamipide in a mouse model of Sjögren’s syndrome. Arthritis Rheum. 2008;58:389–400.
Oka H, Nakano H, Kimata T, Matsuda T, Ozaki S. Effect of rebamipide for the treatment of xerostomia in patients with Sjögren’s syndrome. Progr Med. 2004;24:205–10.
Kohler PF, Winter ME. A quantitative test for xerostomia. The Saxon test, an oral equivalent of the Schirmer test. Arthritis Rheum. 1985;28:1128–32.
Strong B, Farley W, Stern ME, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea. 2005;24:80–5.
Cummins MJ, Papas A, Kammer GM, Fox PC. Treatment of primary Sjögren’s syndrome with low-dose human interferon alfa administered by the oromucosal route: combined phase III results. Arthritis Rheum. 2003;49:585–93.
Smith JK, Siddiqui AA, Modica LA, Dykes R, Simmons C, Schmidt J, et al. Interferon-alpha upregulates gene expression of aquaporin-5 in human parotid glands. J Interferon Cytokine Res. 1999;19:929–35.
Fox RI, Chan E, Benton L, Fong S, Friedlaender M, Howell FV. Treatment of primary Sjögren’s syndrome with hydroxychloroquine. Am J Med. 1988;85:62–7.
Tishler M, Yaron I, Shirazi I, Yaron M. Hydroxychloroquine treatment for primary Sjögren’s syndrome: its effect on salivary and serum inflammatory markers. Ann Rheum Dis. 1999;58:253–6.
Miyawaki S, Nishiyama S, Matoba K. Efficacy of low-dose prednisolone maintenance for saliva production and serological abnormalities in patients with primary Sjögren’s syndrome. Intern Med. 1999;38:938–43.
Zandbelt MM, van den Hoogen FH, de Wilde PC, van den Berg PJ, Schneider HG, van de Putte LB. Reversibility of histological and immunohistological abnormalities in sublabial salivary gland biopsy specimens following treatment with corticosteroids in Sjögren’s syndrome. Ann Rheum Dis. 2001;60:511–3.
Aihara M, Imagawa K, Funakoshi Y, Ohmoto Y, Kikuchi M. Effects of rebamipide on production of several cytokines by human peripheral blood mononuclear cells. Dig Dis Sci. 1998;43:160S–6S.
Yoshikawa T, Naito Y, Tanigawa T, Kondo M. Free radical scavenging activity of the novel anti-ulcer agent rebamipide studied by electron spin resonance. Arzneimittelforschung. 1993;43:363–6.
Urashima H, Okamoto T, Takeji Y, Shinohara H, Fujisawa S. Rebamipide increases the amount of mucin-like substances on the conjunctiva and cornea in the N-acetylcysteine-treated in vivo model. Cornea. 2004;23:613–9.
Ríos JD, Shatos M, Urashima H, Tran H, Dartt DA. OPC-12759 increases proliferation of cultured rat conjunctival goblet cells. Cornea. 2006;25:573–81.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sugai, S., Takahashi, H., Ohta, S. et al. Efficacy and safety of rebamipide for the treatment of dry mouth symptoms in patients with Sjögren’s syndrome: a double-blind placebo-controlled multicenter trial. Mod Rheumatol 19, 114–124 (2009). https://doi.org/10.1007/s10165-008-0141-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10165-008-0141-1