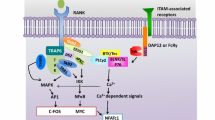
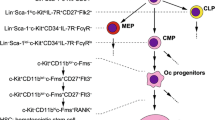
Abstract
Osteoclasts are bone-resorbing multinuclear polykaryon that are essential for bone remodeling and are formed through cell fusion of mononuclear macrophage/monocyte-lineage hematopoietic precursors. In arthritic joints, a large number of activated osteoclasts can be detected, which are suggested to be causative of bone erosion in rheumatoid arthritis. It has been fully established that osteoclastogenesis is critically regulated by several key essential factors, such as M-CSF and RANKL. However, regarding their most characteristic property, i.e., cell fusion to form giant polykaryons, there are still miscellaneous questions to be clarified, although several molecules have been shown to be critically involved in this process. Here we review the latest knowledge about osteoclastogenic cell fusion and novel concepts underlying the characteristic phenomenon. Because cell fusion is a genuine property of mature osteoclasts, modulating this process will become a promising therapeutic tool for bone resorptive disorders in the future.




Similar content being viewed by others
References
Bromley M, Bertfield H, Evanson JM, Woolley DE. Bidirectional erosion of cartilage in rheumatoid knee joints. Ann Rheum Dis. 1985;44:676–81.
Kuratani T, Nagata K, Kukita T, Hotokebuchi T, Nakashima A, Iijima T. Induction of abundant osteoclast-like multinucleated giant cells in adjuvant arthritic rats with accompanying disordered high bone turnover. Histol Histopathol. 1988;13:751–9.
Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8.
Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–49.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42.
Vignery A. Macrophage fusion: are somatic and cancer cells possible partners? Trends Cell Biol. 2005;15:188–93.
Chen EH, Olson EN. Unveiling the mechanisms of cell–cell fusion. Science. 2005;308:369–73.
Nixon B, Aitken RJ, McLaughlin EA. New insights into the molecular mechanisms of sperm-egg interaction. Cell Mol Life Sci. 2007;64:1805–23.
Kaji K, Kudo A. The mechanism of sperm–oocyte fusion in mammals. Reproduction. 2004;127:423–9.
Taylor MV. Muscle differentiation: signaling cell fusion. Curr Biol. 2003;13:R964–6.
Prisk V, Huard J. Muscle injuries and repair: the role of prostaglandins and inflammation. Histol Histopathol. 2003;18:1243–56.
Chen EH, Grote E, Mohler W, Vignery A. Cell–cell fusion. FEBS Lett. 2007;581:2181–93.
Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, Nagata K, Iijima T, Horiuchi M, Matsusaki H, Hieshima K, Yoshie O, Nomiyama H. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med. 2004;200:941–6.
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T. DC-STAMP is essential for cell–cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–51.
Baylies MK, Bate M, Ruiz-Gomez M. Myogenesis: a view from Drosophila. Cell. 1998;93:921–7.
Beckett K, Baylies MK. 3D analysis of founder cell and fusion competent myoblast arrangements outlines a new model of myoblast fusion. Dev Biol. 2007;309:113–25.
Bondesen BA, Jones KA, Glasgow WC, Pavlath GK. Inhibition of myoblast migration by prostacyclin is associated with enhanced cell fusion. FASEB J. 2007;21:3338–45.
Vignery A. Macrophage fusion: the making of osteoclasts and giant cells. J Exp Med. 2005;202:337–40.
Vignery A. Osteoclasts and giant cells: macrophage-macrophage fusion mechanism. Int J Exp Pathol. 2000;81:291–304.
Saginario C, Qian HY, Vignery A. Identification of an inducible surface molecule specific to fusing macrophages. Proc Natl Acad Sci USA. 1995;92:12210–4.
Saginario C, Sterling H, Beckers C, Kobayashi R, Solimena M, Ullu E, Vignery A. MFR, a putative receptor mediating the fusion of macrophages. Mol Cell Biol. 1998;18:6213–23.
Han X, Sterling H, Chen Y, Saginario C, Brown EJ, Frazier WA, Lindberg FP, Vignery A. CD47, a ligand for the macrophage fusion receptor, participates in macrohage multinucleation. J Biol Chem. 2000;275:37984–92.
Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism and disease. Annu Rev Immunol. 1999;17:657–700.
Sterling H, Saginario C, Vignery A. CD44 occupancy prevents macrophage multinucleation. J Cell Biol. 1998;143:837–47.
Ohgimoto S, Tabata N, Suga S, Nishio M, Ohta H, Tsurudome M, Komada H, Kawano M, Watanabe N, Ito Y. Molecular characterization of fusion regulatory protein-1 (FRP-1) that induces multinucleated giant cell formation of monocytes and HIV gp160-mediated cell fusion. FRP-1 and 4F2/CD98 are identical molecules. J Immunol. 1995;155:3585–92.
Cui W, Cuartas E, Ke J, Zhang Q, Einarsson HB, Segdwick JD, Li J, Vignery A. CD200 and its receptor, CD200R, modulate bone mass via the differentiation of osteoclasts. Proc Natl Acad Sci USA. 2007;104:14436–41.
Mbalaviele G, Chen H, Boyce BF, Mundy GR, Yoneda T. The role of cadherin in the generation of multinucleated osteoclasts from mononuclear precursors in murine marrow. J Clin Invest. 1995;95:2757–65.
Lemaire I, Falzoni S, Leduc N, Zhang B, Pellegatti P, Adinolfi E, Chiozzi P, Di Virgilio F. Involvement of the purinergic P2X7 receptor in the formation of multinucleated giant cells. J Immunol. 2006;177:7257–65.
Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, Kang JS, Miyamoto T, Suda T, Lee SK, Pignolo RJ, Koczon-Jaremko B, Lorenzo J, Choi Y. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med. 2006;12:1403–9.
Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci. 2001;58:1189–205.
Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422.
Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–11.
Tarrant JM, Robb L, van Spriel AB, Wright MD. Tetraspanins: molecular organizers of the leukocyte surface. Trends Immunol. 2003;24:610–7.
Wright MD, Moseley GW, van Spriel AB. Tetraspanin microdomains in immune cell signaling and malignant disease. Tissue Antigens. 2004;64:533–42.
Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, Sakagami J, Tada N, Miyazaki S, Kudo A. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet. 2000;24:279–82.
Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science. 2000;287:319–21.
Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, Ryu F, Suzuki K, Kosai K, Inoue K, Ogura A, Okabe M, Mekada E. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 2000;287:321–4.
Tachibana I, Hemler ME. Role of transmembrane 4 superfamily (TM4SF) proteins CD9 and CD81 in muscle cell fusion and myotube maintenance. J Cell Biol. 1999;146:893–904.
Ishibashi T, Ding L, Ikenaka K, Inoue Y, Miyado K, Mekada E, Baba H. Tetraspanin protein CD9 is a novel paranodal component regulating paranodal junctional formation. J Neurosci. 2004;24:96–102.
Ishii M, Iwai K, Koike M, Ohshima S, Kudo-Tanaka E, Ishii T, Mima T, Katada Y, Miyatake K, Uchiyama Y, Saeki Y. RANKL-induced expression of tetraspanin CD9 in lipid raft membrane microdomain is essential for cell fusion during osteoclastogenesis. J Bone Miner Res. 2006;21:965–76.
Yi T, Kim HJ, Cho JY, Woo KM, Ryoo HM, Kim GS, Baek JH. Tetraspanin CD9 regulates osteoclastogenesis via regulation of p44/42 MAPK activity. Biochem Biophys Res Commun. 2006;347:178–84.
Iwai K, Ishii M, Ohshima S, Miyatake K, Saeki Y. Abundant expression of tetraspanin CD9 in activated osteoclasts in ovariectomy-induced osteoporosis and in bone erosions of collagen-induced arthritis. Rheumatol Int. 2008;28:225–31.
Takeda Y, Tachibana I, Miyado K, Kobayashi M, Miyazaki T, Funakoshi T, Kimura H, Yamane H, Saito Y, Goto H, Yoneda T, Yoshida M, Kumagai T, Osaki T, Hayashi S, Kawase I, Mekada E. Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J Cell Biol. 2003;161:945–56.
Iwai K, Ishii M, Ohshima S, Miyatake K, Saeki Y. Expression and function of transmembrane-4 superfamily (tetraspanin) proteins in osteoclasts: reciprocal roles of Tspan-5 and NET-6 during osteoclastogenesis. Allergol Int. 2007;56:457–63.
Tanio Y, Yamazaki H, Kunisada T, Miyake K, Hayashi SI. CD9 molecule expressed on stromal cells is involved in osteoclastogenesis. Exp Hematol. 1999;27:853–9.
Hayashi S, Miyake K, Kinkade PW. The CD9 molecule on stromal cells. Leuk Lymphoma. 2000;38:265–70.
Yamane H, Tachibana I, Takeda Y, Saito Y, Tamura Y, He P, Suzuki M, Shima Y, Yoneda T, Hoshino S, Inoue K, Kijima T, Yoshida M, Kumagai T, Osaki T, Eishi Y, Kawase I. Propionibacterium acnes-induced hepatic granuloma formation is impaired in mice lacking tetraspanin CD9. J Pathol. 2005;206:486–92.
Gordón-Alonso M, Yañez-Mó M, Barreiro O, Alvarez S, Muñoz-Fernández MA, Valenzuela-Fernández A, Sánchez-Madrid F. Tetraspanins CD9 and CD81 modulate HIV-1-induced membrane fusion. J Immunol. 2006;177:5129–37.
Yáñez-Mó M, Alfranca A, Cabañas C, Marazuela M, Tejedor R, Ursa MA, Ashman LK, de Landázuri MO, Sánchez-Madrid F. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with α3β1 integrin localized at endothelial lateral junctions. J Cell Biol. 1998;141:791–804.
Sterk LM, Geuijen CA, van den Berg JG, Claessen N, Weening JJ, Sonnenberg A. Association of the tetraspanin CD151 with the laminin-binding integrins α3β1, α6β1, α6β4 and α7β1 in cells in culture and in vivo. J Cell Sci. 2002;115:1161–73.
Cherukuri A, Shoham T, Sohn HW, Levy S, Brooks S, Carter R, Pierce SK. The tetraspanin CD81 is necessary for partitioning of coligated CD19/CD21-B cell antigen receptor complexes into signaling-active lipid rafts. J Immunol. 2004;172:370–80.
Cherukuri A, Carter RH, Brooks S, Bornmann W, Finn R, Dowd CS, Pierce SK. B cell signaling is regulated by induced palmitoylation of CD81. J Biol Chem. 2004;279:31973–82.
Claas C, Stipp CS, Hemler ME. Evaluation of TM4SF protein complexes and their relation to lipid rafts. J Biol Chem. 2001;276:7974–84.
Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–4.
Kropshofer H, Spindeldreher S, Röhn TA, Platania N, Grygar C, Daniel N, Wölpl A, Langen H, Horejsi V, Vogt AB. Tetraspan microdomains distinct from lipid rafts enrich select peptide-MHC class II complexes. Nat Immunol. 2002;3:61–8.
Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72.
Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911.
Chernomordik LV Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207.
Pike LJ, Han X, Chung K-N, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–88.
Hope HR, Pike LJ. Phosphoinositides and phosphoinositide-utilizing enzymes in detergent-insoluble lipid domains. Mol Biol Cell. 1996;7:843–5.
Muller M, Zschornig O, Ohki S, Arnold K. Fusion, leakage and surface hydrophobicity of vesicles containing phosphoinositides: influence of steric and electrostatic effects. J Membr Biol. 2003;192:33–43.
Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–64.
Acknowledgments
We thank Ms. Kaori Iwai (Department of Clinical Research, Osaka Minami Medical Center) for providing imaging data and helpful discussions. This work was supported by a Grant-in-Aid for the Encouragement of Young Scientists (15790133 and 17790170) from the Ministry of Education, Science, Sports and Culture of Japan, and by a Grant-in-Aid from Takeda Science Foundation, and by a Grant-in-Aid from Kanae Foundation for Socio-Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental movie S1 (mov 3.41 MB)
About this article
Cite this article
Ishii, M., Saeki, Y. Osteoclast cell fusion: mechanisms and molecules. Mod Rheumatol 18, 220–227 (2008). https://doi.org/10.1007/s10165-008-0051-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10165-008-0051-2