Abstract
The characteristic inking behaviour of cephalopods is a secondary defence mechanism that helps them to escape predation. However, although it has been postulated that ink creates a decoy by disrupting the visual information received by the predator, the underlying mechanisms by which ink helps squid to escape from predators remain unknown. Therefore, we observed the inking behaviour of the Japanese pygmy squid (Idiosepius paradoxus). Field observations showed the squid intermittently and linearly ejected ink while rapidly swimming backwards in response to a predator and also changed their bodies to a light colour. This behaviour was then followed by a sudden and sharp change in swimming direction or sometimes a sudden break from swimming with a concurrent change in body colour to black. We also recorded the escape behaviour with inking under captive conditions in response to predatory sculpins, which allowed the successful escape from a predatory attack. Furthermore, the greater the number of ink ejections, the higher the probability that the sculpins would initiate a predatory attack on the ink rather than the squid. Together, these results suggest that squid inking behaviour exhibits a substantial decoy effect on predators and is associated with a series of complex, spatio-temporally regulated behaviours. Digital video images related to the article are available at http://www.momo-p.com/showdetail-e.php?movieid=momo200107ip01a, http://www.momo-p.com/showdetail-e.php?movieid=momo200107ip03a, and http://www.momo-p.com/showdetail-e.php?movieid=momo200107ip04a.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cephalopods exhibit two types of characteristic defence mechanisms: camouflaging their body by changing its colour and texture to match the background and ejecting ink to confuse a predator (Hanlon and Messenger 2018). The former behaviour is known to be the primary defence mechanism that decreases the likelihood of detection by a predator, whereas the latter is regarded as a secondary defence mechanism that allows the individual to escape predation even after an attack has been initiated. However, while there have been a large number of studies on the camouflaging behaviour to date (Hanlon et al. 2008; Hanlon and Messenger 2018 Akkaynak et al. 2013), the mechanism of inking remains poorly understood.
Inking has been proposed to have both chemical and visual effects on predators (Derby 2007), with the ink cloud acting as a decoy for squid and a smokescreen for cuttlefishes and octopuses (Hall 1956; Staudinger et al. 2013; Hanlon and Messenger 2018). The use of ink as a defence mechanism has been proposed based on field observations (Moynihan and Rodaniche 1982; Hanlon and Messenger 1988; Caldwell 2005); however, only few studies have experimentally investigated how ink can confuse the predator and whether it acts as a pseudomorph (decoy) or a smokescreen (Wood et al. 2010; Derby et al. 2013).
Since the pioneering study conducted by Hall (1956) that proposed that squid ink has a decoy effect on predators, few studies have considered this subject area. However, a recent experimental study by Wood et al. (2010) showed that the ink pseudomorph produced by the Caribbean reef squid (Sepioteuthis sepioidea) significantly delays food capture by the predatory fish Haemulon flavolineatum by preventing it from receiving visual and chemical cues; Derby et al. (2013) observed a similar effect in the longfin inshore squid (Doryteuthis pealeii). However, both these studies were conducted under experimental conditions and did not use live squid; thus, it is still unclear whether these effects can also occur in field conditions. Furthermore, although Staudinger et al. (2011) observed the defence behaviours of D. pealeii towards summer flounder (Paralichthys dentatus) and bluefish (Pomatomus saltatrix) in aquaria and noted that these predatory fishes misdirected their attacks after inking by D. pealeii, they did not focus specifically on the inking behaviour and did not test its effectiveness. Therefore, it is still unknown if inking has a decoy effect in cephalopods.
In this study, we investigated whether the inking behaviour of squid has a decoy effect and the mechanism by which predators are misdirected by observing the inking behaviour of the Japanese pygmy squid (Idiosepius paradoxus) in response to a predator mimic in the field, expecting that the squid would perform their natural inking behaviour even though a predator mimic was used. In addition, we conducted an aquarium experiment in which squid were introduced to predatory sculpins (Pseudoblennius spp.) to observe their inking behaviour in the presence of actual predator prey interactions.
Methods
Field experiments
The behaviours of Japanese pygmy squid inhabiting nearshore seagrass beds (approximately 5 m in depth) around the Oki Islands, Shimane Prefecture, Japan (36° 10ʹ N, 133° 16ʹ E) were observed on dives undertaken during May and July 2018. The behaviours were recorded using an action camera (GoPro HERO 4 Silver edition; GoPro Inc., CA, USA) that was mounted on an extension arm (50 cm in length) and had a fishing lure (vibration jig head, 90 mm in length, yellow and red colour) attached at the tip to stimulate escape behaviour in the squid. When a Japanese pygmy squid was found in the water, the camera was moved close to it and all escape behaviours were recorded until the squid exhibited a large change in swimming behaviour (i.e. stopped swimming or sharply changed its swimming course). These behaviours were analysed on the camera display.
Aquarium experiment
Japanese pygmy squid [dorsal mantle length range from 6.8 to 15.4 mm] were collected from seagrass beds in the nearshore waters of the Chita Peninsula (34° 43ʹ N, 136° 58ʹ E), central Honshu, Japan, in July and November 2017 and 2018 using a small drag net. The squid was transported to the laboratory at Oki Marine Biological Station, Japan, where they were maintained in an aquarium (42 × 31 × 16 cm) with a free-flowing system and were fed live prawns (Neocaridina denticulata) ad libitum each day.
Predatory sculpins in the genus Pseudoblennis [total body length (TL) ± SD = 84.65 ± 17.12 mm, n = 8] were collected from seagrass beds in the nearshore waters of the Oki Islands in July and November 2017 and 2018 using a hand net and SCUBA diving. Pseudoblennius sculpins were selected as predators because they prey on Japanese pygmy squid in the field (N. Sato, personal observation). The sculpins were maintained in aquaria (40 × 25 × 30 cm) with a free-flowing system and were fed live prawns (Palaemon sp.) ad libitum each day. The squid and sculpin aquaria were maintained at an ambient temperature of 20–25 °C under a 12-h light/12-h dark cycle.
The behavioural experiments were carried out in aquaria (45 × 20 × 30 cm) that were separated into two areas (predator and observation area) with a black plastic board. A plastic plate (10.0 × 0.6 cm) was placed on the sandy bottom of the observation area to provide a perch for the squid and was encased by a transparent pipe (ϕ6 cm) to encourage the squid to attach to it. Prior to each experiment, a squid was introduced into the pipe in the observation area (30 × 20 × 30 cm) and a sculpin was introduced into the predator area (15 × 20 × 30 cm) and the animals were allowed to acclimatize to the aquarium conditions for at least 1 h before the trials began. The pipe and the black partition were then gently removed once it had been confirmed that the squid had attached to the plate. The behaviours of the squid and sculpin were recorded using a digital video camera (FDR-AX40; Sony, Tokyo, Japan). The trial was stopped soon after a chain of predatory attack events was observed or after 30 min if no such event was observed, and the squid was quickly removed from the aquarium. In total, 148 trials were conducted, in which each squid was used only once and the sculpins were used repeatedly.
Statistical analysis
A generalized linear mixed model (GLMM) with a binomial distribution and logit link function was used to determine whether the target of a predatory attack was influenced by the number of inking events. In this analysis, the targeted object was used as the response variable (1 = squid, 0 = ink cloud) and the ID of the predator and the trial were included as random effects. The significance of the effect of inking frequency was assessed using the Wald test. All analyses were carried out in R version 3.4.2 (R Core Team 2017).
Ethics
These experiments were conducted in accordance with the Shimane University Animal Experimentation Regulations (Shimane University’s Animal Care and Use Committee, H28, H29). In the aquarium experiment, we used the minimum number of animals necessary to achieve the research objectives and allowed the squid and the sculpins to become aware of each other simultaneously rather than forcing a predatory attack. Consequently, there was often no attack within 30 min (65 experiments). A total of 40 squid were eaten during the course of our experiments, but this process was equivalent to what happens in nature and did not result in any unnatural pain or suffering to the squid.
Results
Field observations
All observations were recorded after the diving experimenter moved squid in water from the seagrass leaf edge using a diving pointer. We recorded a total of 58 inking behaviours from 36 individuals (Fig. 1). The squid ejected ink intermittently soon after an escape behaviour was initiated, resulting in 7.6 ± 5.6 (mean ± SD) ink clouds being generated after a sequence of escape behaviours. Before the onset of the escape behaviour, the animals had a darker body colour (98% light brown, 1% yellow and 1% black) in 48 cases and a lighter body colour in 10 cases. Furthermore, we found that a change in body colour occurred more frequently in individuals whose initial body colour was darker (Table 1), and in 36 of 39 cases (92.3%), this darker body changed to a lighter colour, usually soon after the first ink secretion (mean time = 1.46 ± 0.93 s). In all cases, the animals escaped by swimming linearly for a short period of time. They then exhibited a sudden break in swimming in 36 cases (62.1%; defined as ‘S-stop’; Fig. 1a, http://www.momo-p.com/showdetail-e.php?movieid=momo200107ip01a/video S1) or a sharp change in swimming direction in 16 cases (27.6%; defined as a ‘C-change’; Fig. 1b, http://www.momo-p.com/showdetail-e.php?movieid=momo200107ip03a/video S2). In the remaining six cases, we lost track of the squid due to interference by the seagrass beds. Of the 36 cases where animals exhibited an S-stop, 20 (55.6%) showed a colour change from translucent to black. By contrast, of the 16 cases where animals exhibited a C-change, 14 (87.5%) showed no change in body colour, remaining translucent. This difference in body colour change between squid exhibiting an S-stop and C-change was significant (Table 2).
Field observation of escape behaviour in the pygmy squid. Red circle indicates the squid’s location. a Squid suddenly and sharply changed their swimming course ejecting inking (defined as C-change) (http://www.momo-p.com/showdetail-e.php?movieid=momo200107ip01a). b Squid suddenly stopped swimming and turned into dark colour (defined as S-stop) (http://www.momo-p.com/showdetail-e.php?movieid=momo200107ip03a)
Aquarium experiment
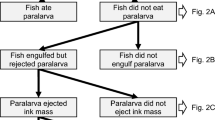
In the aquarium experiment, there was no predatory attack in 21 trials, no inking response in 47 trials (Of these, predatory attack succeeded in 36 trials.) and neither predatory action nor an inking response in 44 trials. Consequently, these 112 trials were removed from further analysis. The sculpins attempted to attack the squid in all 36 of the remaining trials. In 13 of these trials, the sculpins stopped approaching the squid following inking behaviour. In the remaining 23 trials, we were able to observe an entire sequence of prey-predator interactions, i.e. a predatory attack by the sculpin and an escape from this through inking by the squid (Fig. 2, http://www.momo-p.com/showdetail-e.php?movieid=momo200107ip03a/video S3). In these trials, four squid failed to escape from the predatory attack, while the remaining 19 squid (82.6%) successfully escaped. The predatory attack was directed towards the squid more frequently when fewer ink clouds were ejected, with an increase in inking frequency causing a significant change in the predatory target from the squid to the ink clouds (GLMM with binomial error distribution and logit link: n = 49, Wald’s Z = − 2.16, p < 0.05; Fig. 3).
Aquarium observation of escape behaviour in the pygmy squid (http://www.momo-p.com/showdetail-e.php?movieid=momo200107ip04a). a Squid escape ejecting ink clouds when they noticed pre-moving of predatory attack. b Predatory sculpin attacked toward ink
Relationship between the likelihood of a squid or an ink cloud being the target of predatory attacks and the number of ink clouds released. Collected data are indicated by open circles. The solid line shows the predicted values from the generalised linear mixed model with a binomial error distribution and a logit link function
Discussion
To understand the defence mechanism of inking in squid, we observed how they use ink to escape and how their ejected ink confuses the predator. Squid inking has a decoy effect, acting in combination with other behavioural elements (e.g. a timely change in body colour, continuous ink ejection and a sharp turn in swimming direction) to ensure successful escape from predation. Our results suggest that the decoy effect of inking is accomplished through a series of complex, spatio-temporally regulated behaviours rather than simply producing a prey mimic.
When the Japanese pygmy squid swam away from the predator mimics in the field, their chromatophores were retracted, turning their body colour pale. However, because there were few instances where the squid escaped without also exhibiting inking behaviour, it remains unknown if this skin bleaching is always associated with inking behaviour. The divers who recorded the underwater videos in the field study traced the escaping squid via the ejected ink clouds rather than the individuals themselves, suggesting that the sharp contrast between the blackish ink clouds and the less visible translucent bodies of the squid may also direct the predator’s attention towards the decoy. Since predators have a tendency to select more conspicuous prey (Mueller 1971), such a change in body colour would be an effective strategy for driving the predator towards an alternative target.
Both the field and aquarium experiments showed that the squid ejected ink several times while escaping. While some predatory fish were able to attack the prey target even in the presence of ink clouds, many tended to misdirect their targeting as the number of ink clouds increased, exhibiting more frequent attacks on the ink. Thus, this serial ink ejecting behaviour may improve the decoy effect. It has previously been shown that white leghorn chicks that are feeding on conspicuous grains have a decreased ability to detect cryptic grains that are dyed the same colour as the background (Dawkins 1971). In a similar way, the search image of predatory fishes may be dark coloured squid, making the lighter colour far harder to detect. Thus, the camouflage effect that results from cephalopods changing their body pattern and colour according to the background (Hanlon and Messenger 2018) will be reinforced when the predator recognizes the ink clouds as prey.
The field observations showed that the squid escaped from the predators by swimming linearly and producing ink clouds and then suddenly changed their behaviour, exhibiting a C-change or an S-stop (Fig. 4). Before these behaviours were initiated, the predator had already started to chase the prey, presumably by tracing the conspicuous ink clouds rather than the squid itself. Therefore, the predator’s attention would be moved towards the extended line of serial ink clouds, allowing the squid to escape easily from their approach. In the case of the S-stop, the squid changed their body colour to black, causing the predators to mistake them for ink clouds and thus fail target the correct prey. Indeed, we often lost the escaping squid we were watching in the field.
Is this sequential behaviour related to the ink defence specific to I. paradoxus or general among cephalopods? Bush and Robison (2007) have suggested that more active species of oceanic squid, such as Dosidicus gigas, eject a thick cloud, but less active species, such as Chiroteuthis calyx, eject a diffuse cloud. Therefore, it would be important for C. calyx to swim fast. Many coleoid squid might use this complex behaviour to increase the decoy effect because they can change their body colour to a lighter shade while ejecting a dense ink (Hanlon and Messenger 2018). Less active species not having the ability of changing their body colour to a lighter shade would not show complex behaviour to escape from predators, but similar complex behaviour was reported in Octopus vulgaris that swiftly changes its swimming course, ejecting ink in the opposite direction (called ‘fake right, go left’; Hanlon and Messenger 2018).
In secondary defence, some animals emit sounds, such as caterpillars (Bura et al. 2011) and cods (Vester et al. 2004), or chemicals, such as ostracods and shrimp (fluorescent ink) (Haddock et al. 2010) and bombardier beetles (hot chemicals) (Sugiura and Sato 2018), in response to a predator’s attack. However, there is no evidence of a coupled behavioural component to reinforce their defence ability. It is popular in the decoy defence mechanism in the lizard’s autotomy (Bateman and Fleming 2009), but it would not relate with the technique to use the mechanism because that is used automatically after capturing by predators. The complex defence mechanism in cephalopods having a high ability of perception and body colour change is originally evolved to guard their soft body. Therefore, further studies are required to investigate whether these complex escape behaviours are executed as a stereotypical response or on a case-by-case basis, depending on the predator species, background information or a cost–risk analysis.
References
Akkaynak D, Allen JJ, Mäthger LM, Chiao CC, Hanlon RH (2013) Quantification of cuttlefish (Sepia officinalis) camouflage: a study of color and luminance using in situ spectrometry. J Comp Physiol A 199:211–225
Bateman PW, Fleming PA (2009) To cut a long tail short: a review of lizard caudal autotomy studies carried out over the last 20 years. J Zool 277:1–14
Bura VL, Rohwer VG, Martin PR, Yack JE (2011) Whistling in caterpillars (Amorpha juglandis, Bombycoidea): sound-producing mechanism and function. J Exp Biol 214:30–37
Bush SL, Robison BH (2007) Ink utilization by mesopelagic squid. Mar Biol 152:485–494
Caldwell RL (2005) An observation of inking behavior protecting adult Octopus bocki from predation by green turtle (Chelonia mydas) hatchlings. Pac Sci 59:69–73
Dawkins M (1971) Perceptual changes in chicks: another look at the ‘search image’ concept. Anim Behav 19:566–574
Derby CD (2007) Escape by inking and secreting: marine molluscs avoid predators through a rich array of chemicals and mechanisms. Biol Bull 213:274–289
Derby CD, Tottempudi M, Love-Chezem T, Wolfe LS (2013) Ink from longfin inshore squid, Doryteuthis pealeii, as a chemical and visual defense against two predatory fishes, summer flounder, Paralichthys dentatus, and sea catfish, Ariopsis felis. Biol Bull 225:152–160
Haddock S, Moline M, Case J (2010) Bioluminescence in the Sea. Annu Rev Mar Sci 2:443–493
Hall DNF (1956) Ink ejection by Cephalopoda. Nature 177:663
Hanlon RT, Messenger JB (1988) Adaptive coloration in young cuttlefish (Sepia officinalis L.): the morphology and development of body patterns and their relation to behaviour. Philos Trans R Soc B 320:437–487
Hanlon RT, Messenger JB (2018) Cephalopod Behaviour, 2nd edn. Cambridge University Press, New York
Hanlon RT, Chiao CC, Mäthger LM, Barbosa A, Buresch KC, Chubb C (2008) Cephalopod dynamic camouflage: bridging the continuum between background matching and disruptive coloration. Philos Trans R Soc B 364:429–437
Moynihan M, Rodaniche AF (1982) The behavior and natural history of the Caribbean reef squid Sepioteuthis sepioidea with a consideration of social, signal, and defensive patterns for difficult and dangerous environments. Adv Ethol 25:1–150
Mueller HC (1971) Oddity and specific searching image more important than conspicuousness in prey selection. Nature 233:345–346
R Core Team (2017) R: a language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing. See https://www.R-project.org/
Staudinger MD, Hanlon RT, Juanes F (2011) Primary and secondary defences of squid to cruising and ambush fish predators: variable tactics and their survival value. Anim Behav 81:585–594
Staudinger MD, Buresch KC, Mäthger LM, Fry C, McAnulty S, Ulmer KM, Hanlon RH (2013) Defensive responses of cuttlefish to different teleost predators. Biol Bull 225:161–174
Sugiura S, Sato T (2018) Successful escape of bombardier beetles from predator digestive systems. Biol Lett 14:20170647
Vester IH, Folkow LP, Blix AS (2004) Click sounds produced by cod (Gadus morhua). J Acoust Soc Am 115:914–919
Wood JB, Maynard AE, Lawlor AG, Sawyer EK, Simmons DM, Pennoyer KE, Derby CD (2010) Caribbean reef squid, Sepioteuthis sepioidea, use ink as a defense against predatory French grunts, Haemulon flavolineatum. J Exp Mar Biol Ecol 388:20–27
Acknowledgements
We thank Hiroki Ono for care of the squid. This study was supported by a grant from Kakenhi (18K05786 to N.S.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MOV 34684 kb)
Supplementary material 2 (MOV 48387 kb)
Supplementary material 3 (MOV 85489 kb)
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hikidi, Y., Hirohashi, N., Kasugai, T. et al. An elaborate behavioural sequence reinforces the decoy effect of ink during predatory attacks on squid. J Ethol 38, 155–160 (2020). https://doi.org/10.1007/s10164-020-00640-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-020-00640-8