Abstract
Habitat preference is thought to be genetically programmed in fishes. However, fishes can choose habitat based on their personal experience of an environment. We investigated whether the environment in which fish are raised affects habitat preference in red sea bream Pagrus major juveniles, and tested if the formed preference lasts until later life stages. Juveniles were reared in tanks with a substrate of either sand or artificial seaweed for 40 days. Naive fish were raised without either type of substrate. In the preference test, individual fish were allowed to choose either a sand or artificial seaweed microhabitat. The tested fish were then kept in barren tanks, and similar tests conducted again on days 30 and 100. Sand and seaweed treatment fish preferred the corresponding habitat immediately after the rearing treatment, whereas naive fish did not exhibit any preference. These preferences were maintained when fish were tested on day 30, but not on day 100. The present study suggests that habitat preference is acquired through the rearing environment at the nursery stage, and that this preference lasts for at least 30 days. The formation of habitat preference should help juveniles to choose an optimal microhabitat in a fluctuating environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most marine fishes migrate from an offshore pelagic to coastal neritic environment at the early life stage, after which settled juveniles need to choose a suitable habitat, with sufficient food and the lowest predation risk, as a nursery ground. Field surveys and experiments indicate that fish often show a preference for certain environments (Stuntz et al. 2001; De la Morinière et al. 2002; Burfeind et al. 2009). The preference for an environment is often genetically programmed in fishes. For example, hatchery reared juveniles of two flatfish species exhibited s preference for habitats with a rougher structure over a smoother sand substratum, despite the fact that they had never experienced these environments before (Stoner and Titgen 2003). Also, some fishes show an innate preference for darker environments due to an anxious condition (Maximino et al. 2007). The preference for a habitat is formed by natural selection and is thus assumed to be an innate behavioral characteristic of each species.
However, fish can change their behavioral characteristics through their experience of the environment in which they live (Frost et al. 2007; Bhat et al. 2015; Takahashi and Masuda 2018). For example, white sea bream Diplodus sargus juveniles reared with bricks used a brick as a refuge more quickly than naïve individuals when attacked by a dummy predator (D’Anna et al. 2012). In our previous study, Japanese flounder Paralichthys olivaceus juveniles, which were reared with pseudo-predator attack for 2 weeks, changed their behavior to feed staying on the bottom (Takahashi et al. 2013). These studies suggest that fish can acquire a suitable behavioral characteristic depending on their surroundings, and thus habitat preference as a behavioral characteristic might also be affected by the living environment.
Studies have revealed that habitat preferences are also fine-tuned through experience in a particular habitat. Anemonefish Amphiprion percula at the settlement stage prefer habitat experienced at the larval stage (Dixon et al. 2014). Juvenile Dascyllus aruanus occurred more frequently amongst coral from which they had been collected than other coral species (Sale 1971). The formation of preference for a living environment may help juveniles, which are at risk of predation and starvation, to choose a suitable habitat they have previously experienced. However, there are very few studies on the formation of habitat preference for the living environment in fish. In the few studies mentioned above, most focused on the development of habitat preference for a short period, but did not investigate for how long the preference was maintained.
In the present study, we investigated whether the habitat preference of red sea bream Pagrus major juveniles is formed depending on their experience of the nursery environment, and, if they form a preference, the preference would last until a later stage. P. major are pelagic in offshore areas at the larval stage, and then they settle in coastal neritic habitats at a standard length (SL) of approximately 15 mm (Tanaka 1985; Tsukamoto et al. 1989). After migration, settled juveniles live in a sandy bottom habitat with features such as eelgrass in sandy areas or boulders in more stony ones (Tanaka 1985; Kudoh et al. 1999; Tomioka et al. 2011). Settled juveniles live in coastal areas to form their territories from a size of approximately 40 mm total length (TL; = ca. 30–35 mm SL) (Kudoh et al. 1999), and live in bottom structure habitat at this size. Juveniles choose suitable habitats as their nursery grounds. They live in the nursery ground for a few months, and then migrate to offshore areas at ca. 90 mm TL (= ca. 75–80 mm SL) (Tsukamoto et al. 1989). Thus, the preference for a habitat found at the settlement stage should be kept for a while, but might be lost at the migration stage.
We tested two hypotheses using red sea bream juveniles: (1) prior rearing environment affects habitat preference, and (2) the effect of prior experience on habitat preference lasts for only a certain period. In the present study, the bottom of rearing tanks for the test groups was composed of either sand or seaweed, both types of habitat preferred by natural populations of sea bream (Kudoh and Yamaoka 1998; Kudoh et al. 1999). The treatment started at the recruitment stage (ca. 15 mm SL) and continued until the early coastal stage (ca. 30 mm SL). The retention of preference was tested at the early coastal stage, late coastal stage (ca. 60 mm SL) and migration stage (ca. 90 mm SL).
Materials and methods
Fish
Fertilized P. major eggs (approximate 20,000 eggs), purchased from Marua Suisan (Ehime Prefecture, Japan), were transported to the Maizuru Fisheries Research Station, Kyoto University, and kept in a 500-L transparent polyethylene stock tank under natural light. Water in the tank was maintained by exchange at a rate of 4 L/min; the tanks were aerated. Seawater used for the rearing was pumped up from near the Maizuru Fisheries Research Station and filtered through a fine mesh. After hatching on 17 May 2013, larvae were provided with rotifer Brachionus plicatilis sp. complex (approximately 5 individuals/ml), brine shrimp Artemia sp. nauplii (approximately 2 individuals/ml), and dry pellets (Otohime B1, C2, S2; Marubeni Nisshin); quantities were adjusted according to growth. Fish were reared for up to 40 days post hatching (DPH) in the stock tank, and some were used for the following experiments.
Treatment
Two 30-L circular transparent polycarbonate tanks were used for the sand treatment and the seaweed treatment. The side of each tank was covered with blue vinyl sheeting to prevent disturbance from outside. Water circulation and aeration in these tanks were similar to those of the stock tank; the tanks were covered with a net to prevent the fish from jumping out. The treatment tanks were placed adjacent to each other and were provided with the same source of seawater to minimize differences in environmental conditions other than those of the treatment itself.
In the sand treatment, artificial ceramic sand (Micros Ceramic MS-0; Norra; diameter < 0.5 mm) was spread evenly to a thickness of approximately 3 cm on the tank bottom. In the seaweed treatment, artificial green seaweed (length 100 cm, length of leaf-shaped area 20 cm; New Kinran; Tanaka Sanjiro) was cut into 20-cm-long pieces and used to cover the entire bottom of the tank. Thirty fish (40 DPH) were captured from the stock tank and were introduced into each treatment tank on 27 June 2013. Fish were fed pellets twice daily, and the tank bottoms were gently siphoned to remove deposits every 3–5 days. Fish were reared for 40 days in each treatment tank, i.e., the fish were 80 DPH at the end of the treatment, at which time they were tested for habitat preference.
Preference test
A blue rectangular tank (length × width × height: 170 × 78 × 38 cm) was used as the test tank for habitat preference (Fig. 1). The tank was filled with seawater to a depth of 30 cm, and the water was changed every ten trials. Sand and seaweed areas were located on either side of the tank. In the sand area, ceramic sand was placed to a thickness of 1 cm in six polypropylene cases (length × width × height: 32 × 24 × 1 cm for each and arranged in a 2 × 3 design, i.e., 64 × 72 cm in total). In the seaweed area, the artificial seaweed was spread over the entire area (64 × 72 cm). The test tank was separated from the observer by a black sheet. The side of the tank for the different substrates was switched every ten trials.
The first habitat preference tests [day-0 test, when the fish were 80 or 81 DPH; body length 31 ± 3 mm (mean ± SD)] were conducted on 7 and 8 August 2013. Fourteen individuals (data for one individual of the seaweed treatment were lost due to a recording failure) from each treatment, as well as those from the stock tank, were used, the latter being tested as naive fish without experience of sand or seaweed. A single fish was captured from one of these tanks using a hand net, and placed in a 1000-mL transparent beaker. Then, the beaker was immersed into the center of the test tank with its opening at the surface until the fish began to swim into the tank. The behavior of each fish was recorded for 20 min using a video camera (HC-V100 M; Panasonic) set above the test tank. The fish were then captured, transferred into a beaker, and photographed. Body length was determined from the photograph using Image J software (Open Source, Public Domain, NIH).
Tested fish were then introduced into one of three transparent polyethylene 100-L tanks of their respective treatments, i.e., sand, seaweed, or naïve treatment. The conditions of the holding tanks were similar to those of the treatment tanks in terms of water circulation and feeding, but no bottom structure was provided in any treatment. After 30 days of rearing in these tanks, the second habitat preference tests (day-30 test, when the fish were 110–111 DPH; body length 57 ± 5 mm) were conducted for each individual in the same manner as in the first trials (each treatment, n = 14). Tested fish were temporarily put into buckets and then returned to each 100-L holding tank. The fish in each treatment were maintained for another 70 days. The third preferences test (day-100 test, when the fish were 180 DPH; body length 92 ± 9 mm) was conducted for only treatment fish (sand treatment fish, n = 5; seaweed treatment fish, n = 8) because, most naïve fish died before this test due to an accident.
Analysis
The first choice of habitat in the experimental tank was judged from recorded video images for each individual based on which bottom structure area the fish first entered. For each treatment, the habitat choice for each treatment fish was compared with a 50% by binomial test. If a fish did not enter either the sand and seaweed, its data were excluded from the analysis of the first choice test.
In addition, video images were analyzed by determining the position of fish at 1-s intervals; a score of +1 was given to fish in the sand area, −1 for fish in the seaweed area, and 0 for fish in the middle area. The sum of the scores for each fish recorded for 1200 s was calculated and divided by 1200, and the values were used as a habitat preference index, i.e., fish that showed a preference for sand had a maximum score of 1, and those preferring seaweed had a minimum score of −1. The habitat preference index for each treatment on each test day was compared to 0, i.e., the value expected when no preference was shown for sand or seaweed. A one-sample Wilcoxon test was used because the data were non-homoscedastic. These analyses were conducted in R statistical software (version 3.4.2).
Ethical notes
All experiments were performed according to the regulations on animal experimentation of Kyoto University. A minimum number of fish was used to test the hypothesis. After the experiment, the fish were kept in the laboratory as broodstock.
Results
Behavioral observation
Most of the tested fish in all the treatments chose the habitat by slow movement. Thus, we consider that there was no major effect of motor activity for habitat choice between treatments.
Day-0 test
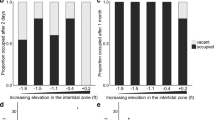
For the first choice of habitat, 11 fish of the sand treatment chose the sand area, no fish chose the seaweed area (Fig. 2a), and three fish did not enter either the sand or seaweed area. Meanwhile, all of seaweed treatment fish (n = 13) entered the seaweed area. Both types of treatment fish chose significantly more often the environment that they had experienced during the treatment period (sand treatment, p < 0.001; seaweed treatment, p < 0.001). Among the naive fish, the sand area were chosen by eight fish and seaweed area by five fish; there was no statistically significant preference for either habitat (p = 0.58).
The median habitat preference index was 0.46 (interquartile range 0.09–0.65) for sand treatment fish and − 0.74 (− 0.56 to − 0.83) for seaweed treatment fish; they were both significantly different from 0 (sand treatment V = 63, p < 0.01, seaweed treatment V = 0, p < 0.001; Fig. 3a), i.e., the sand treatment fish preferred sand and the seagrass treatment fish preferred seagrass. In addition, the preference index of naive fish from the stock tank was 0.12 (− 0.2 to 0.38), which was not significantly different from 0 (V = 53, p = 0.6).
Habitat preference index, with a higher value representing a preference for the sand habitat and lower value representing a preference for the seaweed habitat, in red sea bream juveniles reared either with sand, seaweed, or no-substrate on the tank bottom and tested at a day-0, b day-30, and c day-100 post-treatment. Black squares represent medians, error bars represent the first and third interquartile range, and open circles represent raw data for each individual
Day-30 test
All fish of the sand treatment (n = 14) chose the sand area in the first choice experiment, and this choice was statistically significant (p < 0.001; Fig. 2b). For the first choice of seaweed fish, ten fish chose the seaweed area, three fish the sand area, and one fish chose neither the sand nor the seaweed area; these fish showed a tendency to choose the seaweed area with a marginal significance (p = 0.09). Naive fish did not show a preference for either habitat: eight fish chose sand, and five fish seaweed (p = 0.58).
The habitat preference index was 0.67 (0.45–0.75) for the sand treatment and − 0.45 (− 0.05 to − 0.8) for the seaweed treatment, both significantly different from 0 (sand treatment V = 105, p < 0.001, seaweed treatment V = 16, p < 0.05; Fig. 3b). The index for naive fish was 0.09 (0.15–0.75), and did not significantly differ from 0 (V = 68, p = 0.3).
Day-100 test
For the sand treatment fish (n = 5), two chose the sand area and three the seaweed area. For the seaweed treatment, two chose sand and six the seaweed area. There was no preference for either habitat in fish of either treatment (sand treatment, p = 1.00; seaweed treatment, p = 0.29; Fig. 2c). The habitat preference index was − 0.81 (− 0.27 to − 0.89) for the sand treatment and − 0.84 (− 0.43 to − 0.99) for the seaweed treatment, neither of which was significantly different from 0 (sand treatment V = 2, p > 0.1, seaweed treatment V = 5, p > 0.05; Fig. 3c).
Discussion
Fish reared in either treatment environment clearly preferred the corresponding habitat in the day-0 test. This indicates that preference for habitat was formed through the fishes’ experience of their rearing habitat. Some studies have indicated that habitat preference is determined by natal habitat experience (Davis and Stamps 2004). In fish, natal habitat preference has been reported in coral fishes (Arvedlund et al. 1999; Dixon et al. 2014). A preference for natal habitat might help in the quick and efficient dispersion of individuals (Stamps 2001; Davis and Stamps 2004). In the present study, habitat preference of P. major was formed for its nursery habitat, as is the case in natal habitat preference in past studies. Adult P. major spawn eggs in offshore spawning grounds, after which hatched larvae are transported by tidal currents to the coastal zone in approximately 1 month (Tanaka 1985). Thus, the recruitment environment differs greatly from the natal habitat. For such species, it is adaptive for the fish to form a habitat preference for the nursery ground habitat at the juvenile stage, rather than for the natal habitat.
Fish often show an innate habitat preference for particular structures and environments, as mentioned above. An innate preference for a microhabitat is useful for the prompt choice of a habitat, especially under stable conditions. However, in the present study, such a preference was absent in the naive fish, indicating that P. major juveniles at this stage of post-settlement do not have an innate species’ preference for the tested environments, but rather acquire it from experience. In P. major, the hatched larvae are transported to the coastal zone over their long life in the pelagic zone (Tanaka 1985). There is always uncertainty regarding which type of habitat larvae can recruit into, and the coastal environment into which they recruit is variable. In such circumstances, habitat choice based on the evaluation of environment on its own would be advantageous for these fish rather than an innately fixed preference.
Fish often exhibit a preference for a place with rewards such as food and other addictive substances (e.g., cocaine and amphetamine) (Mathur et al. 2011; Millot et al. 2014). Such preferences are considered the result of associative learning of conditioned spatial cues with rewards. Juveniles in the present study, however, did not undergo conditioning that included any particular reward, and thus the formation of habitat preference is considered to be a type of non-associative learning for that particular environment. The habitat preference for each treatment fish might have been formed by their familiarity with each nursery environment. Some studies have shown that animals that are familiar with their surroundings can gain a benefit for survival (Daly et al. 1990; Clarke et al. 1993). In coyote Canus latrans, individuals in familiar environments could detect novel stimuli and treat them with caution (Windberg 1996). Brown (2001) found that crimson spotted rainbowfish Melanotaenia duboulayi became familiar with an experimental environment after 3 weeks of rearing in a tank. In the present study, the treatment tanks had sufficient food and no predators, and thus familiarity might have been nurtured in such environments. If a habitat where juveniles are recruited has sufficient food and a low risk of predation, it can be predicted that they will stay in this habitat rather than take the risk of moving. Thus, the preference for a familiar habitat may play a role in the survival strategy of settled juveniles.
Treatment fish continued to show a preference for the corresponding rearing habitat even after being reared in a barren environment for 30 days. Memories acquired at the early life stage generally last for a long period (Moretz et al. 2007; Salvanes et al. 2007; Arnold and Taborsky 2010). For example, the density of conspecifics in early life can influence the sociality of guppies Poecilia reticulata in later stages (Chapman et al. 2008). In the present study, we used fish at the early life stage; consequently the preference that they formed for a habitat was maintained for some time, despite some of them having had the experience of no environmental structure prior to the preference test. There is a danger of predation and starvation during early life stages. The retention of the preference for a particular suitable habitat benefits survival by reducing the cost of searching for and estimating the potential of a habitat. Meanwhile, the coastal environment can often fluctuate, and the suitability of a habitat may vary during the development of an organism, e.g., due to predator immigration and prey disappearance. If it is difficult to change the habitat preference formed at the early life stage, juveniles cannot move and may then have to endure a suboptimal habitat. Further study is required to investigate the shift of habitat preference when a habitat deteriorates.
The habitat preference that had been formed disappeared by day 100, although it should be noted that the statistical power was less for this timepoint than for those of the previous two tests. Possible explanations for the disappearance of habitat preference include the loss of memory since the nursery phase and the ‘overwriting’ of preference for the barren tank. Another possibility is that a shift of habitat preference may be innately programmed; P. major juveniles migrate from coastal shores to deeper areas in autumn (Tanaka 1985), and the day-100 test was conducted at the time of offshore migration. If habitat shift is genetically programmed by a factor related to seasonal changes, such as lower water temperature, the experience-formed preference may be lost. In any case, the present study implies that habitat preference is at least partially acquired rather than fully innately programmed.
The present study revealed that habitat choice of fish can be formed based on their living environment. The results suggest that researchers should be careful when experimenting with and observing hatchery-reared fish to evaluate the habitat preference of a target species. Further studies using various species are required to investigate the formation of habitat preference in fish. The formation of habitat preference may be useful to improve the behavior of seed fish during release in stock enhancement programs. Naive hatchery-reared fish often fail to choose an appropriate habitat (Stuntz et al. 2001; Kawabata et al. 2011), resulting in a high mortality rate after release. The life skills of seed fish may be improved by rearing them in a suitable environment to nurture an appropriate habitat preference for survival in their future natural environment.
Change history
20 December 2019
The article Nurture is above nature: nursery experience determines habitat preference of red sea bream <Emphasis Type="Italic">Pagrus major</Emphasis> juveniles, written by Kohji Takahashi and Reiji Masuda, was originally published Online First without Open Access. After publication in volume 37, issue 3, page 317–323 the author decided to opt for Open Choice and to make the article an Open Access publication. Therefore, the copyright of the article has been changed to © The Author(s) 2019 and the article is forthwith distributed under the terms of the Creative Commons Attribution 4.0 International License (<ExternalRef><RefSource>http://creativecommons.org/licenses/by/4.0/</RefSource><RefTarget Address="http://creativecommons.org/licenses/by/4.0/" TargetType="URL"/></ExternalRef>), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
Arnold C, Taborsky B (2010) Social experience in early ontogeny has lasting effects on social skills in cooperatively breeding cichlids. Anim Behav 79:621–630
Arvedlund M, McCormick MI, Fautin DG, Bildsoe M (1999) Host recognition and possible imprinting in the anemonefish Amphiprion melanopus (Pisces: Pomacentridae). Mar Ecol Prog Ser 188:207–218
Bhat A, Greulich MG, Martins EP (2015) Behavioral plasticity in response to environmental manipulation among zebrafish (Danio rerio) populations. PLOS ONE 10:e0125097
Brown C (2001) Familiarity with the test environment improves escape responses in the crimson spotted rainbowfish, Melanotaenia duboulayi. Anim Cognit 4:109–113
Burfeind DD, Tibbetts IR, Udy JW (2009) Habitat preference of three common fishes for seagrass, Caulerpa taxofolia, and unvegetated substrate in Moreton Bay, Australia. Environ Biol Fish 84:317–322
Chapman BB, Ward AJW, Krause JK (2008) Schooling and learning: early social environment predicts social learning ability in the guppy, Poecilia reticulata. Anim Behav 76:923–929
Clarke MF, Burke Da Silva K, Lair H, Pocklington R, Kramer DL, Mclaughlin RL (1993) Site familiarity affects escape behaviour of the eastern chipmunk, Tamias striatus. Oikos 66:533–537
D’Anna G, Giacalone VM, Vega Fernández T, Vaccaro AM, Pipitone C, Mirto S, Mazzola S, Badalamenti F (2012) Effects of predator and shelter conditioning on hatchery-reared white sea bream Diplodus sargus (L., 1758) released at sea. Aquaculture 356–357:91–97
Daly M, Wilson MI, Behrends PR, Jacobs LF (1990) Characteristics of kangaroo rats, Dipodomys merriamu, associated with differential predation risk. Anim Behav 40:380–389
Davis JM, Stamps JA (2004) The effect of natal experience on habitat preferences. Trend Ecol Evol 19:411–416
De la Morinière EC, Pollux BJA, Nagelkerken I, Van der Velde G (2002) Post-settlement life cycle migration patterns and habitat preference of coral reef fish that use seagrass and mangrove habitats as nurseries. Estuar Coast Shelf Sci 55:309–321
Dixon DL, Jones GP, Munday PL, Planes S, Pratchett MS, Thorrold SR (2014) Experimental evaluation of imprinting and the role innate preference plays in habitat selection in a coral reef fish. Oecologia 174:99–107
Frost AJ, Winrow-Giffen A, Ashley PJ, Sneddon LU (2007) Plasticity in animal personality: does prior experience alter the degree of boldness? Proc R Soc B Biol Sci 274:333–339
Kawabata Y, Asami K, Kobayashi M, Sato T, Okuzawa K, Yamada H, Yoseda K, Arai N (2011) Effect of shelter acclimation on the post-release survival of hatchery-reared black-spot tuskfish Choerodon schoenleinii: laboratory experiments using the reef-resident predator white-streaked grouper Epinephelus ongus. Fish Sci 77:79–85
Kudoh T, Yamaoka K (1998) Territory establishment location and foraging behaviour of juveniles of red sea bream Pagrus major and crimson sea bream Evynnis japonica. Nippon Suisan Gakkaishi 64:16–25
Kudoh T, Suetomo K, Yamaoka K (1999) Distribution and behaviour of wild and artificially reared juveniles of red sea bream Pagrus major at Morode Cove in Ehime Prefecture. Nippon Suisan Gakkaishi 65:230–240 (In Japanese with English abstract)
Mathur P, Lau B, Guo S (2011) Conditioned place preference behaveor in zebrafish. Nat Prot 6:338–345
Maximino C, Marques T, Dias F, Cortes FV, Taccolini IB, Pereira PM et al (2007) A comparative analysis of the preference for dark environments in five teleosts. Int J Comp Psychol 20(3):51–367
Millot S, Cerqueira M, Castanheira MF, Øverli Ø, Martins CIM, Oliveira RF (2014) Use of conditioned place preference/avoidance tests to assess affective states in fish. Appl Anim Behav Sci 154:104–111
Moretz JA, Martins EP, Robinson BD (2007) The effects of early and adult social environment on zebrafish (Danio rerio) behavior. Environ Biol Fish 80:91–101
Sale PF (1971) Apparent effect of prior experience on a habitat preference exhibited by the reef fish, Dascyllus aruanus (Pisces: Pomacentridae). Anim Behav 19:251–256
Salvanes AGV, Moberg O, Braithwaite VA (2007) Effects of early experience on group behaviour in fish. Anim Behav 74:805–811
Stamps JA (2001) Habitat selection by dispersers: integrating proximate and ultimate approaches. In: Clobert J (ed) Dispersal. Oxford University Press, London, pp 230–242
Stoner AW, Titgen RH (2003) Biological structures and bottom type influences habitat choices made by Alaska flatfishes. J Exp Mar Biol Ecol 292:43–59
Stuntz GW, Levin PS, Minello TJ (2001) Selection of estuarine nursery habitats by wild-caught and hatchery-reared juvenile red drum in laboratory mesocosms. Environ Biol Fish 61:305–313
Takahashi K, Masuda R (2018) Net-chasing training improves the behavioral characteristics of hatchery-reared red sea bream (Pagrus major) juveniles. Can J Fish Aquat Sci 75:861–867
Takahashi K, Masuda R, Yamashita Y (2013) Bottom feeding and net chasing improve foraging behavior in hatchery-reared Japanese flounder Paralichthys olivaceus juveniles for stocking. Fish Sci 79:55–60
Tanaka M (1985) Factors affecting the inshore migration of pelagic larval and demersal juvenile red sea bream Pagrus major to a nursery ground. Trans Am Fish Soc 114:471–477
Tomioka K, Ohmae S, Abe F, Yamaoka K (2011) Territory and feeding habit of juvenile red sea bream at littoral boulder area. Kuroshio Sci 4:159–167 (In Japanese with English abstract)
Tsukamoto K, Kuwada H, Hirokawa J, Oya M, Sekiya S, Fujimoto H, Imaizumi K (1989) Size-dependent mortality of red sea bream, Pagrus major, juveniles released with fluorescent otolith-tags in News Bay, Japan. J Fish Biol 35:59–69
Windberg LA (1996) Coyote responses to visual and olfactory stimuli related to familiarity with an area. Can J Zool 74:2248–2253
Acknowledgments
The present study was funded by a grant-in-aid for Japan Society for the Promotion of Science Fellows and Early-Career Scientists (KAKENHI grant nos. 15J06124 and 18K14512, respectively), and a Sasakawa Scientific research grant from the Japan Science Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to a retrospective Open Access order.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Takahashi, K., Masuda, R. Nurture is above nature: nursery experience determines habitat preference of red sea bream Pagrus major juveniles. J Ethol 37, 317–323 (2019). https://doi.org/10.1007/s10164-019-00605-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-019-00605-6