Abstract
Males of the hermit crab Pagurus filholi show precopulatory guarding behavior in which a male grasps the shell of a mature female with his left cheliped before copulation. Hermit crabs were most common in rocky intertidal areas with cobbles and boulders, while many guarding pairs were observed on fronds of brown algae such as Sargassum confusum in Hakodate Bay, Japan. We examined three hypotheses explaining why the guarding pairs were most common on algae; (1) aggregation place for mature males and females to find mates, (2) avoidance of male–male combat, and (3) avoidance of predators. If solitary males and females climbed up algae, then many guarding pairs were observed after pairing, but only guarding pairs climbed up the algae after removing all crabs. Experiments in aquariums showed that the disturbance rate for guarding pairs due to male–male competition was lower on the algae than in boulder and rocky flat areas, and few disturbances were observed by predatory crabs in all habitat types. These results suggest that the guarding males climb up the fronds of algae to sequester guarded females from rival males and avoid male–male combat. This behavior could be considered as a male counter tactic against indirect female choice mediated by sex pheromones in which females release sex pheromones while guarded, attracting many rival males and inducing male–male competition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual selection usually comprises two important processes; male–male competition and female choice, and there is a controversy as to whether male–male competition facilitates or hampers female choice (Wong and Candolin 2005). These two components of sexual selection have traditionally been viewed as complementary in their effects, and competitive interactions between rival males are thought to be beneficial for females (Wiley and Poston 1996). On the other hand, studies from the viewpoint of sexual conflict suggest that male–male competition need not facilitate female choice. Superior males in competition increase their own mating opportunities by excluding rival males (Moore et al. 2001; Andersson et al. 2002) and this may occur even if their actions reduce female fitness (Sih et al. 2002; Wong and Candolin 2005). The most effective mechanism for excluding rival males seems to be mate monopolization in space and time (Shuster and Wade 2003; Jormalainen 2007). Precopulatory mate guarding behavior is a typical example of male mate monopolization, which is a common mating strategy when female receptivity for copulation is short and the rate of encountering receptive females is low (Jormalainen 1998). The relative ability of males to monopolize receptive females leads to assuring their paternity and thus increases their fitness.
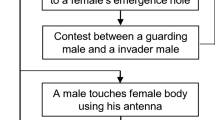
In pagurid hermit crabs, precopulatory mate guarding behavior is frequently observed, in which a male grasps the edge of a shell occupied by a ripe female with his left cheliped (Imafuku 1986) and drags the shell for up to 5 days in the case of Pagurus filholi (Goshima et al. 1998). The rate of encounter with receptive females that include non-ovigerous and ovigerous females with late-stage eggs is generally low except for just the beginning of the spawning season (Goshima et al. 1998). As most females are smaller than males in body size (Yoshino et al. 2002; Goshima et al. 2006), females hardly resist the guarding attempt by males. Therefore, active female mate choice may be restricted by this male coercive behavior, and male–male competition only seems to work as a sexual selection process in attaining copulation. However, females release sex pheromones while guarded, which induces male–male combat over the guarded females (Okamura and Goshima 2010). As a result of combat, the larger males end up guarding the females. Consequently, females choose males indirectly by exploiting male–male competition induced by sex pheromones under male coercive behavior (Yamanoi et al. 2006; Okamura and Goshima 2010). Against male coercive behavior, females set the condition for competition among males by delaying copulation and releasing sex pheromones, increasing the chances of mating with successful competitors, and so indirectly choose larger and stronger mates (Okamura and Goshima 2010). In this case male–male competition indirectly facilitates female choice.
Against such female choice, a male antagonistic and effective strategy is to detect receptive females quickly and monopolize them in space to exclude rival males before the above mentioned indirect female choice process begins. Hiding the receptive female from rival males is most likely a tactic for avoiding male–male competition, because guarding pairs themselves are easily distinguished among competitive males and then tend to induce male–male combat (Andersson 1994; Okamura and Goshima 2010). For example, male fiddler crabs invite wandering receptive females into their burrow and then seal the burrow entrance from within to mate and spawn, avoiding detection by rival males and excluding them (Murai et al. 1987; Goshima and Murai 1988). Furthermore, in the sandstone-boring isopod, guarding cohabitation in the female’s burrow lasts up to 5 months (Murata and Wada 2002), which may also serve to avoid rival males.
In the hermit crab Pagurus filholi, the guarding pairs are often observed on fronds of leafy brown algae during the reproductive season (S. Goshima, personal observation). This behavior is thought to be a special habitat use pattern, only observed in the reproductive season, which suggests that some benefit may be gained for the guarding pairs by climbing the fronds. In this paper, we examine three hypotheses explaining why the guarding pairs were most common on the fronds of algae: they climbed up algae (1) to find mates; the algae fronds are aggregation places for mature solitary males and females to mate, (2) to avoid male–male competition over receptive females, and (3) to avoid potential predators. We first examined either solitary crabs or guarding pairs that had climbed up algae to test hypothesis 1. If the solitary males and females had climbed up to find mates, then many guarding pairs might be observed on the algae after pairing, because the precopulatory guarding behavior lasts for several days (Goshima et al. 1998). On the other hand, if guarding pairs climbed up, then the aggregation hypothesis will not be accepted. We then performed several experiments to examine the other two hypotheses. In hypothesis 2 (avoidance of competing males), the tactic of hiding receptive females on the algae may play an important role in precopulatory mate guarding, as already mentioned. In hypothesis 3 (avoidance of predators), some studies found that guarding pairs are more conspicuous, less mobile, and have a higher risk of predation than solitary individuals (Magnhagen 1991; Andersson 1994; Jormalainen 1998; Wellborn and Cothran 2007). For example, in fish predation treatments for a freshwater amphipod species, guarding pairs were consumed about twice as frequently as solitary individuals (Cothran 2004). If this is the case in the hermit crab, males may perform precopulatory guarding behavior on the algae with less visibility among leafy fronds, leading to a lower predation risk.
Materials and methods
Our study site was a flat intertidal rocky shore at Kattoshi, Hakodate Bay, southern Hokkaido, Japan (41º44′34″N, 140º36′08″E), where various sizes of cobbles and boulders are scattered on the shore. More than five hermit crab species belonging to the family Paguridae are distributed at Kattoshi, among them, Pagurus filholi is one of the most abundant species (Goshima et al. 1996). They are distributed throughout the tidal flat and usually hide themselves around and under the cobbles and boulders during low tide (Goshima et al. 2006). The breeding season of Pagurus filholi ranged from March to July at our study site, and precopulatory mate guarding is often observed during the season, lasting for up to 5 days until spawning (Goshima et al. 1998). The guarding male separates from the guarded female just after the copulation and spawning process. The mean duration of copulation is about 30 s, and the spawning occurs during the postcopulatory guarding phase for about 30 min, during which the female lays eggs while in her shell (Minouchi and Goshima 1998). The duration of the copulation and spawning is considerably shorter than whole precopulatory guarding process, and difficult to observe. Therefore, almost all behavior criteria were obtained from precopulatory guarding behaviors in the present study.
All hermit crabs for the following experiments were collected from the Kattoshi shore. Sampling of the hermit crabs was conducted by lifting or turning over the cobbles and boulders by hand during low tide from March to July in 2005 and 2006. We measured the shield length (SL; calcified anterior portion of the cephalothorax) as the measure of body size.
Distribution of hermit crabs in different habitat types
The flat rocky shore of the study site at Kattoshi was mainly composed of three habitat types; cobbles and boulders, bare flat rock, and flat rock with brown algae such as Sargassum confusum and Coccophore langsdorfii. We placed the same number of 2–6 quadrats (50 × 50 cm) randomly in each habitat type during every field visit from March to July to examine the distribution pattern of solitary crabs and guarding pairs among the habitat types. All hermit crabs in the quadrats were collected, sexed, and the females were determined as ovigerous or non-ovigerous. A total of 90 quadrats were sampled in each habitat type. To examine the distribution pattern of the hermit crabs, we compared mean densities of the hermit crabs and mean numbers of guarding pairs among the three habitat types by 1-way ANOVA. As response variables did not conform to the ANOVA assumption of homogeneous variances even after log- or square root-transformation, we adopted a post hoc test based on a sandwich estimator with no assumption of homogeneous variances for multiple comparisons. We also compared proportions of solitary males, solitary female and guarding pairs among different habitat types using a χ 2 test to examine whether guarding pairs showed any particular distribution pattern.
Hypothesis 1
Algae are the aggregation place of mature solitary males and females for mating
Experiment A: Are algae the aggregation place of mature solitary males and females?
We examined hypothesis 1 using Sargassum weed (Sargassum confusum) in the field. Ten separate weeds, which were about 30 cm in diameter as viewed from above and about 30 cm in height, were selected haphazardly, and all hermit crabs were removed and sexed. After removing individuals we started the experiment at 7:00 a.m. in June, which is the mid spawning month (Goshima et al. 1998), and then counted individuals that climbed the fronds of algae every 30 min for the first 3 h. The last count was done after 6 h. Hermit crabs on the algae were defined as solitary (single males or females) or guarding pairs in which a male was grasping the shell of a female with his left cheliped, and we checked which type of hermit crabs, solitary individuals or guarding pairs, climbed up the algae.
Hypothesis 2
Guarding pairs climb up algae to avoid male–male competition
Experiment B: Do guarding pairs climb up algae to avoid male–male competition?
Experiment B-1: If males adopt a hiding tactic by climbing algae, then the presence of rival solitary males would induce the guarding males to climb up algae to avoid such rivals. To test whether chemical cues and/or a visible stimulus from rival males alters the behavior of the guarding males, we conducted the following male–male competition experiment. Since in the field it was difficult to conduct experiments concerning chemical cues that needed control of water movement, we did the experiments in aquariums. We prepared acrylic resin aquariums (45 cm × 30 cm × 30 cm high) with seawater and gravel on the bottom in which we placed a natural alga of Sargassum confusum of about 25 cm height. The alga was anchored into bottom sediment at one side of the aquarium, and a transparent acrylic resin enclosure (8 cm × 8 cm × 10 cm high) with slits was placed at the other side of the tank. The slits enabled exchange of seawater between the inside of the enclosure and the rest of the tank, and the transparent enclosure enabled the focal guarding pairs to recognize rival males visibly. A guarding pair was placed in the center of each aquarium, and after 5 min we introduced a male that had guarded a female in the field into the enclosure as a rival male. For the rival males, we set three body sizes relative to the guarding male; rival solitary male < guarding male, solitary male = guarding male, and solitary male > guarding male. As a control, we introduced no solitary male into the enclosure. A total of 80 sets of the experiment were conducted; 20 sets each for one of the three body size ratios between solitary and guarding males and another 20 sets for the control. All experiments were conducted at room temperatures of about 20 °C, using natural seawater from the study area. We checked whether the guarding pair was on the fronds of the alga every 30 min for 2 h. The total number of pairs found on the algae fronds was compared among the three body size ratio groups and the control without rival males using a χ 2 test.
Experiment B-2: Another experiment in male–male competition was conducted where individuals of a guarding pair and a solitary rival male were allowed physical contact. In this experiment, we tested whether the behavior of climbing up algae was effective in achieving precopulatory mate guarding. We prepared acrylic resin aquariums (45 cm × 30 cm × 30 cm high) with seawater and gravel on the bottom in which we mimicked one of the three typical environments of the study site; a natural alga Sargassum confusum of about 25 cm height, a natural cobble of about 20 cm diameter and 15 cm height (mimic of the boulders), or no structure within (mimic of the flat rock). We put a guarding pair into each aquarium, and after 5 min we introduced a male that had guarded a female in the field into the aquarium as a rival male. For the rival males, we set three body sizes relative to the guarding male; rival solitary male < guarding male, solitary male = guarding male, and solitary male > guarding male. We checked whether the guarding pair was disturbed and separated by the rival male or intact every 10 min for 2 h. A total of 135 sets of the experiment were conducted; 15 sets each for one of the three environment mimics and one of the three body size ratios between solitary and guarding males. Residual percentages of the guarding pairs were compared among the three environment mimics within each body size ratio group by Cox’s proportional hazard model. Actual copulation is of course a better measure of reproductive success, but copulation duration is very short for the hermit crab (30 ± 27 SD s, Minouchi and Goshima 1998) and it was difficult to confirm all of the copulations in this experiment. Precopulatory guarding behavior leads to high probability of actual copulation (Goshima et al. 1998), and we measured performance of the precopulatory guarding behavior as an indicator of the reproductive success.
Hypothesis 3
Guarding pairs climb up algae to avoid potential predators
Experiment C: Do guarding pairs climb up algae to avoid potential predators?
Experiment C-1: If presence of predators induces guarding pairs to climb up algae to avoid them, we expect guarding pairs to respond accordingly in trials of presence/absence of potential predators. There are several potential predators for the hermit crabs in Kattoshi: among them the most commonly observed one is a grapsid crab, Gaetice depressus (Yoshino et al. 2002). We prepared acrylic resin aquariums (45 cm × 30 cm × 30 cm high) with seawater and gravel on the bottom in which we placed a natural alga into the sediment at one side of the aquarium. On the opposite side of the tank, a transparent acrylic resin enclosure (8 cm × 8 cm × 10 cm high) with slits was placed. The slits enabled seawater exchange between the enclosure and the rest of the tank, and the transparent enclosure enabled the focal guarding pairs to recognize predatory crabs visibly. A guarding pair was placed at the center of each aquarium, and then a potential predatory crab (starved for 2 weeks before the experiment) was introduced into the enclosure. For a control, we introduced no crab into the enclosure. We checked whether the guarding pair was on the fronds of the alga every 30 min for 2 h. The experiment was repeated a total of 24 times, where 12 repetitions included a predatory crab and the other 12 sets without crabs were the control. Each predatory crab was used only once. The number of pairs that climbed up algae fronds was compared between groups with and without predatory crabs by Fisher’s exact probability test.
Experiment C-2: Our second experiment testing for predator avoidance was conducted allowing physical contact between a guarding pair and a potential predatory crab. We prepared acrylic resin aquariums (45 cm × 30 cm × 30 cm high) as previously described, in which we mimicked one of the three typical environments of the study site; a natural alga Sargassum confusum of about 25 cm height, a natural cobble of about 20 cm diameter and 15 cm height (mimic of the boulders), or no structure (mimic of the flat rock) within. We put a guarding pair into each aquarium, and then introduced a predatory crab that had been starved for 2 weeks before the experiment into the enclosure. We checked whether the guarding pair was disturbed and separated by the predatory crab or intact every 10 min for 2 h. A total of 39 repetitions were conducted; 13 repetitions of each for one of the three environment mimics. Residual percentages of the guarding pairs were compared among three environment mimics by Cox’s proportional hazard model.
Results
Distribution of hermit crabs in different habitat types
We collected a total of 10,174 hermit crabs, and detected a distinctive distribution pattern among habitat types (Fig. 1). Almost all hermit crabs (98 % of the total crabs) were distributed in the cobble and boulder area with a mean density of 110.5 individuals 0.25 m−2, while 2 % (2.3 crabs 0.25 m−2) were on the fronds of algae, and only 0.2 % (0.3 crabs 0.25 m−2) were in the bare flat rock area (Fig. 1a). There was a significant difference in mean density among habitat types (1-way ANOVA, F 2,267 = 74.791, P < 0.0001). Post-hoc contrasts demonstrated more hermit crabs in the algae than on the flat rock (P < 0.002) and both were far fewer than in the boulder area (P < 0.001 for both; Fig. 1a). While 34 precopulatory guarding pairs (38 % of the total pairs) were distributed in the cobble and boulder area, 54 pairs (61 %) were on the algae, and only 1 pair (1 %) was in the bare flat rock area (Fig. 1b). Significant difference was also detected among the habitat types (1-way ANOVA, F 2,267 = 13.139, P < 0.0001), with 0.4 pairs 0.25 m−2 in the boulders indistinguishable from the 0.6 pairs on the algae (post hoc test, P = 0.136) but both higher than the 0.01 pairs in the flat rock area (P < 0.05 and P < 0.01, respectively). More than 50 % of the hermit crabs collected on the algae were guarding pairs, and the others were solitary males (13 %) and solitary females (36 %). On the other hand, hermit crabs in the boulder areas were composed of only 0.7 % guarding pairs, 22 % solitary males, and 77 % solitary females. Significant difference was also detected in the proportion of solitary males, solitary females and guarding pairs between the boulder area and algae fronds (χ 2 test, χ 2 = 3145.2, df = 2, P < 0.0001). These results indicate that most hermit crabs inhabit the cobble and boulder area, while many precopulatory guarding pairs are on the algae and in the boulders area, and the algae in particular are predominantly inhabited by the guarding pairs.
Mean densities (a) and number of guarding pairs of the hermit crab (b) in three different habitat types; cobbles and boulders, algae, and flat rock areas. Vertical bars indicate +SD. Mean densities differed significantly among the habitat types (1-way ANOVA, F 2,267 = 74.791, P < 0.0001), and a significantly higher proportion of guarding pairs were found on the algae than expected on the basis of percentage of inhabiting number of the hermit crabs on the algae habitat (binomial test, z = 80.052, P < 0.001)
Hypothesis 1
Algae are the aggregation place of mature solitary males and females for mating
Experiment A: Are algae the aggregation place of mature solitary males and females?
Just before experiment A, we removed a total of 13 guarding pairs from 10 algae while solitary individuals were not observed. Figure 2 shows changes in the number of hermit crabs that climbed up immediately after the algae were emptied, in which the newcomers increased with time; however, these newcomer individuals were all paired and no single males or females were observed. After 6 h, a similar total number of guarding pairs (12 pairs) was recovered from the previously manipulated algae, and almost no crabs descended from the algae within 6 h. These results indicate that the algae were not an aggregation place for mature solitary males and females for mating.
Hypothesis 2
Guarding pairs climb up algae to avoid male–male competition
Experiment B: Do guarding pairs climb up algae to avoid male–male competition?
Experiment B-1: Table 1 shows the number of climbing pairs on the algae in each time period with a rival male of each body size ratio group or without a rival male in the enclosure. No pairs descended from the alga fronds after climbing during 120 min observation, and there was no significant difference in the total number of the pairs climbing on the algae among the groups with and without rival males (χ 2 test, χ 2 = 4.262, df = 3, 0.2 < P < 0.3).
Experiment B-2: Figure 3 shows residual percentages of guarding pairs among three environmental mimics in each body size ratio between solitary and guarding males. In the body size ratio of solitary male < guarding male group, there was no significant difference between alga and boulder mimics (Cox’s proportional hazard model, z = 0.930, P = 0.352), and also no difference between alga and flat rock mimics (no structure) (z = 0.899, P = 0.369) (Fig. 3a). In the body size ratio of solitary male = guarding male group, there was significant difference between alga and boulder mimics (z = 2.204, P = 0.028) (Fig. 3b), and also significant difference between alga and flat rock mimics (z = 2.436, P = 0.015). In the body size ratio of solitary male > guarding male group, there was significant difference between alga and boulder mimics (z = 4.329, P < 0.001), and also significant difference between alga and flat rock mimics (z = 3.985, P < 0.001) (Fig. 3c). These results indicate that the guarding pairs on the algae had a tendency to remain undisturbed by rival solitary males and they could keep pairing longer than those in other environmental conditions, particularly for the guarding males similar in body size to the rival males.
Residual percentages of guarding pairs on algae with time in treatments of different body size ratios between solitary males and guarding males: solitary male < guarding male (a); solitary male = guarding male (b); solitary male > guarding male (c). No significant difference was detected between alga and boulder mimics (Cox’s proportional hazard model, z = 0.930, P = 0.352) and between alga and flat rock mimics (z = 0.899, P = 0.369) in the body size ratio of solitary male < guarding male group. Significant differences were detected between alga and boulder mimics (z = 2.204, P = 0.028) and between alga and flat rock mimics (z = 2.436, P = 0.015) in the body size ratio of solitary male = guarding male group, and between alga and boulder mimics (z = 4.329, P < 0.001) and between alga and flat rock mimics (z = 3.985, P < 0.001) in the body size ratio of solitary male > guarding male group
Hypothesis 3
Guarding pairs climb up algae to avoid potential predators
Experiment C: Do guarding pairs climb up algae to avoid potential predators?
Experiment C-1: In the trials testing whether the presence of predators in the enclosure induced guarding pairs to climb up algae to avoid them, only one guarding pair among 12 pairs climbed up an alga at 60 min after the start of the experiment, and also only one pair climbed up at 30 min in the control with no predatory crab. There was no significant difference between the two groups (Fisher’s exact probability test, P = 0.739).
Experiment C-2: In the trials allowing direct physical contact between a guarding pair and a predatory crab, the number of the guarding pairs intact at 120 min was 12, 11, and 11 pairs among initial 13 pairs in the boulders, algae, and flat rock mimics, respectively. There was no significant difference in the residual number of the guarding pairs between algae and boulder mimics (Cox’s proportional hazard model, z = −0.586, P = 0.558), and also no difference between algae and flat rock mimics (z = 0.042, P = 0.967). These results indicate that the guarding pairs were not disturbed by the predatory crabs.
Discussion
We examined three possible hypotheses explaining why the guarding pairs were common on the algae, and among them, two hypotheses, (1) aggregation place for mature solitary males and females to find mates, and (3) avoidance of predators, were not plausible. Hypothesis 1 was rejected in the present study, because no single males and females climbed up the algae. Only guarding pairs approached the algae where all the hermit crabs had been removed, although both single crabs and guarding pairs were observed on the algae in the distribution pattern research (Fig. 1). The guarded female lays eggs just after copulation during postcopulatory guarding, and the male then separates from the female (Minouchi and Goshima 1998). The observed single individuals might be pair-dissolved crabs just after spawning. An alternative explanation on the origin of the observed solitary males might be that competing solitary males climb up algae to take over mature females guarded by males. If solitary males often climbed up to take over, we might expect to see such males during 6 h observations in experiment A. However, no solitary individuals were detected, suggesting the observed single individuals might be pair-dissolved crabs. Climbing up algae would be decided by guarding males because guarded females are passively dragged by the males during precopulatory guarding (Goshima et al. 1998; Minouchi and Goshima 1998).
Potential predators of the hermit crabs include several grapsid crabs: Gaetice depressus, Hemigrapsus sanguineus and H. penicillatus, all found in the present study site (Yoshino et al. 2002; Mima et al. 2003). However, infrequent observation of hermit crabs that had been preyed upon suggests that the predation risk might be low or not effective enough to dissolve pairs in the present study site, which is consistent with the results testing hypothesis 3 (experiment C-2), in which the pairs persisted in precopulatory guarding even though the potential predators were allowed direct contact.
Hypothesis 2 (male–male competition avoidance) seemed to fit best the results of our experiments. When no direct contact was allowed between the guarding pair and the rival male (experiment B-1, enabled water exchange), there was no significant difference in the climbing rate between groups with and without a rival male present. This result does not always mean that the presence of rival males does not induce guarding pairs to climb the algae, but that they likely adopt the tactic of climbing up the algae just after making a pair, since about one fourth of the experimental pairs climbed up the algae irrespective of the presence or absence of rival males.
On the other hand, when direct contact was allowed, the disturbance rate of the guarding pairs was significantly lower on the algae than in the mimics of rocky flat or boulder areas (experiment B-2). Larger guarding males showed lower disturbance rates than single rival males even in the rocky or boulder areas, indicating that larger males would achieve precopulatory guarding anywhere, irrespective of the habitat type, while guarding males similar to or smaller than single rival males would show higher performance rates of guarding on the algae compared with the other habitats. Rival single males seem to have difficulty in disrupting the guarding pairs on the algae, particularly when it comes to guarding males with similar body size to the rival males. Therefore, the most parsimonious conclusion that can be drawn from our analysis is that the guarding males climb up algae to achieve precopulatory mate guarding in less crowded areas where they can avoid rival challengers. The sequestering and hiding behavior of receptive females from competing males suggests a type of scramble competition between males over females in the hermit crab, which may be advantageous because it increases male mating success. However, the story seems to be more complicated as follows.
Female physical resistance is commonly observed against male mating attempts in many animals (Jormalainen 1998, 2007; Wellborn and Cothran 2007). The role of female resistance is explained by two different aspects; minimizing naturally selected costs of pairing (Jormalainen 1998; Chapman et al. 2003) and female mate choice through selective resistance (Sparkes et al. 2002; Cordero and Everhard 2003; Kokko 2005). However, almost no female resistance is observed in male precopulatory mate guarding attempts in the hermit crab except when the female is similar to or larger than the attempting male (Minouchi and Goshima 1998; Goshima et al. 2006). As sexual dimorphism in body size is usually observed in the hermit crab in which males are larger than females (Yoshino et al. 2002; Goshima et al. 2006), female resistance does not lead to rejection of male attempts to secure precopulatory guarding. The females passively accept guarding by the males, and then adopt antagonistic behavior by releasing sex pheromones while guarded (Yamanoi et al. 2006; Okamura and Goshima 2010). The released pheromones induce male–male combat between the guarding males and solitary rival males, and the larger male ends up guarding the female. This is an indirect female choice mechanism which exploits male–male competition (Okamura and Goshima 2010).
Males also adopt a counter tactic against the above indirect female choice. The guarding males climb up algae of less crowded areas to avoid male–male competition. The leafy algae do not only affect visible recognition of guarding pairs by rival males among algae fronds, but also keep a given distance to contain the action radius of female sex pheromones to the rival males beneath. This synergy leads to consistency in duration of guarding pairs and probably contributes to fertilization success of the guarding males. The climbing tactic exhibited by guarding males could be a way of reducing the ability of the guarded females to attract competing males through sex pheromone activity. Indeed, this is an example of sexually antagonistic coevolution exhibited by the hermit crab, predicted to rise under sexual conflict (Arnqvist and Rowe 2005).
We finally refer to the controversy of whether male–male competition facilitates or hampers female choice (Wong and Candolin 2005). As a facilitating component of the competitive interactions between the guarding males and rival single males, we can conclude that indirect female choice, which is induced by the female sex pheromones, is beneficial for the females (Okamura and Goshima 2010). While the scenario in which male–male competition turns out to have negative impacts to indirect female choice is that the climbing behavior conducted by the guarding males excludes rival males that females may otherwise prefer. Therefore, male–male competition has both facilitating and hampering effects on female choice, and its generating force would be the sexual conflict that arises from males and females seeking to maximize their respective fitness returns (Arnqvist and Rowe 2005). Sexual conflict plays an important role in generating various reproductive behaviors exhibited by both male and female hermit crabs. Further studies of hermit crab behavior from the view point of sexual conflict might help shed light on the origin and purpose of various other behaviors, a rewarding area for the enthusiastic empiricist.
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton and Oxford
Andersson S, Pryke SR, Ornborg J, Lawes MJ, Andersson M (2002) Multiple receivers, multiple ornaments, and trade-off between agonistic and epigamic signaling in a widowbird. Am Nat 160:683–691
Arnqvist G, Rowe L (2005) Sexual conflict. Princeton University Press, Princeton and Oxford
Chapman T, Arnqvist G, Bangham J, Rowe L (2003) Sexual conflict. Trend Ecol Evol 18:41–47
Cordero C, Everhard WG (2003) Female choice of sexually antagonistic male adaptations: a critical review of some current research. J Evol Biol 16:1–6
Cothran R (2004) Precopulatory mate guarding affects predation risk in two freshwater amphipod species. Anim Behav 68:1133–1138
Goshima S, Murai M (1988) Mating investment of male fiddler crabs, Uca lactea. Anim Behav 36:1249–1251
Goshima S, Wada S, Ohmori H (1996) Reproductive biology of the hermit crab Pagurus nigrofascia (Anomura: Paguridae). Crust Res 25:86–92
Goshima S, Kawashima T, Wada S (1998) Mate choice by males of hermit crab Pagurus filholi: do males assess ripeness and/or fecundity of females? Ecol Res 13:151–161
Goshima S, Minouchi S, Yoshino K, Wada S (2006) Size assortative mating by the hermit crab Pagurus filholi (Decapoda: Anomura: Paguridae). In: Asakura A (ed) Biology of Anomura II. Crust Res, Special Number 6: 87–94
Imafuku M (1986) Sexual discrimination in the hermit crab Pagurus geminus. J Ethol 4:39–47
Jormalainen V (1998) Precopulatory mate guarding in crustaceans: male competitive strategy and intersexual conflict. Quart Rev Biol 73:275–304
Jormalainen V (2007) Mating strategies in isopods. From mate monopolization to conflicts. In: Duffy JE, Thiel M (eds) Evolutionary ecology of social and sexual systems. Crustaceans as model organisms. Oxford University Press, New York, pp 167–190
Kokko H (2005) Treat’em mean, keep’em (sometimes) keen: evolution of female preferences for dominant and coercive males. Evol Ecol 19:123–135
Magnhagen C (1991) Predation risk as a cost of reproduction. Trend Ecol Evol 6:183–186
Mima A, Wada S, Goshima S (2003) Antipredator defence of the hermit crab Pagurus filholi induced by predatory crabs. Oikos 102:104–110
Minouchi S, Goshima S (1998) Effect of male/female size ratio on mating behavior of the hermit crab Pagurus filholi (Anomura: Paguridae) under experimental conditions. J Crust Biol 18:710–716
Moore AJ, Gowaty PA, Wallin WG, Moore PJ (2001) Sexual conflict and the evolution of female mate choice and male social dominance. Proc R Soc Lond B 268:517–523
Murai M, Goshima S, Henmi Y (1987) Analysis of the mating system of the fiddler crab, Uca lactea. Anim Behav 35:1334–1342
Murata Y, Wada K (2002) Population and reproductive biology of an intertidal sandstone-boring isopod, Sphaeroma wadai Nunomura, 1994. J Natur Hist 36:25–35
Okamura S, Goshima S (2010) Indirect female choice mediated by sex pheromones in the hermit crab Pagurus filholi. J Ethol 28:323–329
Shuster SM, Wade MJ (2003) Mating systems and strategies. Princeton University Press, Princeton
Sih A, Lauer M, Krupa JJ (2002) Path analysis and the relative importance of male-female conflict, female choice and male-male competition in waterstriders. Anim Behav 63:1079–1089
Sparkes TC, Keogh DP, Orsburn TJ (2002) Female resistance and mating outcomes in a stream-dwelling isopod: effects of male energy reserves and mating history. Behaviour 139:875–895
Wellborn GA, Cothran RD (2007) Ecology and evolution of mating behavior in freshwater amphipods. In: Duffy JE, Thiel M (eds) Evolutionary ecology of social and sexual systems. Crustaceans as model organisms. Oxford University Press, New York, pp 147–166
Wiley RH, Poston J (1996) Indirect mate choice, competition for mates, and coevolution of the sexes. Evolution 50:1371–1381
Wong BBM, Candolin U (2005) How is female mate choice affected by male competition? Biol Rev 80:559–571
Yamanoi T, Yoshino K, Kon K, Goshima S (2006) Delayed copulation as a means of female choice by the hermit crab Pagurus filholi. J Ethol 24:213–218
Yoshino K, Goshima S, Nakao S (2002) Temporal reproductive patterns within a breeding season of the hermit crab Pagurus filholi: effects of crab size and shell species. Mar Biol 141:1069–1075
Acknowledgments
We thank R. C. Lombardo for his comments for improvement on earlier versions of the manuscript. Two anonymous referees provided thoughtful suggestions to improve the manuscript. We also appreciate the constructive discussions of members of the Laboratory of Benthos, Graduate School of Fisheries Sciences, Hokkaido University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kawaminami, T., Goshima, S. Guarding males of the hermit crab climb up algae to avoid male–male competition. J Ethol 33, 25–33 (2015). https://doi.org/10.1007/s10164-014-0411-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-014-0411-7