Abstract
The question why females in many species mate with several males (polyandry) has engaged the interest of evolutionary biologists for many years, and many studies have been conducted on the nature of the benefits that the females gain from polyandry. To understand the variation of female mating rates among species and populations it is indispensable to test the prediction that females of more polyandrous populations experience larger fitness benefit than those of less polyandrous populations. We compared the fitness components of two strains of the adzuki bean beetle Callosobruchus chinensis that have genetically different female mating rates. We measured the number of hatched eggs of once-copulated females and twice-copulated females in each strain. The statistical interaction for the number of hatched eggs between the number of matings and strains was determined. The increase in the number of hatched eggs is larger for the lower mating-rate strain than for the higher mating rate strain. This means that females of the lower mating-rate strain would have larger fitness gain from polyandry than those of the higher mating-rate strain. The actual mating rates of females did not reflect female interests in adzuki bean beetles, suggesting they are affected by sexual conflict.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Females of many animal species mate with several males (polyandry), and female mating rates (the number of males that a female mates with; the degree of polyandry) vary among species (Birkhead and Møller 1998). This is inconsistent with the classic proposition that reproductive success of females does not depend on number of mates (Bateman 1948). This inconsistency has induced many studies on the nature of benefits that females gain from polyandry (Hosken and Stockley 2003), and several possible benefits have been suggested. For example, it is suggested that polyandrous females gain benefits from nutritional ejaculates (Gwynne 1997; Vahed 1998), from avoidance of fertilization with genetically incompatible sperms (Zeh and Zeh 1996, 1997; Tregenza and Wedell 1998), and/or from choosing genetically good sperms (Yasui 1997, 1998; Newcomer et al. 1999; Jennions and Petrie 2000).
The reason variation of female mating rates among species and populations is maintained is not fully understood, however. The benefits of polyandry have usually been tested by comparison of artificially monandrous females and polyandrous females in the same population (Arnqvist and Nilsson 2000). To explain the variation in female mating rates observed among species and populations, however, it is necessary to show that variation of female mating rate among species or populations reflects the variation in the fitness payoff of polyandry—the females of more polyandrous species or populations gain greater fitness benefits from polyandry than those of the less polyandrous species or populations (Vahed 1998; Torres-Vila et al. 2004).
To test the prediction, it is ideal to compare not the species but the populations among which the female mating rates are different, because effects of possible confounding factors among populations would be much smaller than those among species. In the adzuki bean beetle Callosobruchus chinensis (Linne), there are the populations, or strains, that have genetically different female mating rates (Miyatake and Matsumura 2004; Harano and Miyatake 2005). Harano and Miyatake (2006) reported that inter-strain variation in female re-mating rate was primarily attributable to female traits, suggesting genetic variation in female receptivity to re-mating in the adzuki bean beetle.
We used two strains of C. chinensis with different female mating rates—a low mating-rate strain (jC strain, which we call the “low strain”, here) and a high mating-rate strain (isC strain, which we call the “high strain”, here). Females were given the opportunity to mate with one male or two males in each strain and the fitness components were measured.
When we compare fitness components we must consider the sampling bias that potentially exists in this kind of experiment (Torres-Vila et al. 2004; Harano et al. 2006) because all of the females that were given opportunities to re-mate do not necessarily accept the males. In the high strain (isC strain) of the adzuki bean beetle, Harano et al. (2006) found that the average body size of the females that accepted re-mating was larger than for females that refused re-mating. They also suggested that the significant cost of re-mating was detected when the sampling bias was considered whereas it was not detected when the sampling bias was not considered (Harano et al. 2006). To consider this body size bias, we used ANCOVA with female body size as a covariate. If the different female mating rates between strains reflects the different fitness payoff of polyandry, two conditions are satisfied at the same time. First, the statistical interaction for fitness components between the number of copulations and strains should be detected. Second, the difference between the average fitness component of the twice-mated females and that of the once-mated females in the high strain should be larger than the corresponding difference in the low strain.
Materials and methods
Beetles
Two strains of C. chinensis were used in the experiments. One was the jC strain which was collected in Kyoto, Japan, in 1940 (Utida 1941a, b), and has been maintained in the laboratory for over 60 years. The second was the isC strain collected in Okinawa, Japan, in 1997 (Yanagi and Miyatake 2003). In C. chinensis, the acceptability to the female of the male mating attempt is known to depend on a variety of conditions. For example, the female re-mating rate (the proportion of the females that re-mated when they were given a one-hour opportunity to re-mate) varies according to the time between matings, the time of day, and the presence of food and water (Harano and Miyatake 2005; personal observation). The re-mating rate of the isC strain was, however, either higher than that of the jC strain or not significantly different from that of the jC strain under all conditions (Harano and Miyatake 2005; personal observation). For example, 29.6% (16/54) of the jC females re-mated whereas 52.8% (28/ 53) of the isC females re-mated under conditions in which their first matings were allowed on the day of their eclosion and re-matings were allowed (for 1 h) 5 days after the eclosion (the time of day was 1800–1900 hours JST, 14 light:10 dark with the light on at 1000 hours JST) (personal observation). We therefore used the jC strain as the “low strain” and the isC strain as the “high strain”. The mating behavior of the adzuki bean beetle is as follows. When the males recognize the females they chase them and try to penetrate female genital openings. Copulation continues for approximately 40 s. All stock cultures were reared at 25°C with 14 light:10 dark (light on at 1000 hours JST) conditions. They were fed on adzuki beans, Vigna angularis, during the larval stage. After eclosion, adults were given neither food nor water. These are standard conditions under which these strains are cultured. All experiments were conducted at 25°C with 14 light:10 dark. The environments in which the two strains had been maintained were not exactly the same (Utida 1941a, b; Yanagi and Miyatake 2003; Harano and Miyatake 2005) but they were cultured under similar conditions (at 25–30°C, with adzuki beans, and without water and food during the adult stage) as far as we are aware.
Adult beetles randomly chosen from the stock cultures were transferred to Petri dishes (height: 2.0 cm, diameter: 9.0 cm) with approximately 0.5 g adzuki beans per adult, and left to oviposit for a day. After 3 weeks, adzuki beans containing only one egg were transferred to a 48-well plate with one bean per well. This procedure ensured that emerging individuals remained virgins. Females that emerged within 24 h and males that emerged within 5 days were randomly chosen and used for the experiment.
Mating experiment
The fitness components of twice-copulated females, in each strain, were measured as follows. Virgin females were put into 5-mL plastic bottles with virgin males. Females that copulated within 10 min were transferred to a single well in a 48-well plate without beans, in isolation from other individuals. Females that did not copulate within 10 min were excluded from further study. After 5 days, females that had copulated once were again transferred to 5-mL plastic bottles with a virgin male. The reason second mating was investigated 5 days after the first is that re-mating rate, hence sample size, was lower if the interval was not sufficient (Harano and Miyatake 2005) and a sufficient sample size cannot be achieved. Each female that copulated within 1 h was transferred to a Petri dish (height: 1.5 cm, diameter: 6.0 cm) with 20 g adzuki beans and left to oviposit until her death. Females that did not copulate within 1 h were excluded from further study. Females were maintained until their death, and adult life span (days from eclosion to death) was recorded except for two females, which were not properly recorded. Body size was measured as the length of the elytra to the nearest 0.01 mm, after death. Two weeks after the death of the female, the total number of eggs a female laid (fecundity) and the total number of hatched eggs were counted. The egg-hatching rate, or fertility, was calculated as the number of hatched eggs divided by the fecundity. For each mating the male was selected from the same strain as the female. The percentage of females that copulated at the first mating opportunity in the low stain and the high strain were 72.7% (96/132) and 89.7% (104/116), respectively; those that copulated at the second mating opportunity were 32.1 (26/81) and 40.0% (24/60), respectively. The reason the re-mating rate of the high strain in this experiment was lower than the re-mating rate described above (52.8%) could be the different conditions of the re-mating opportunities; in this experiment, the time allowed for the first mating opportunity was only 10 min and the re-mating treatment was conducted between 1500 and 2100 hours JST. Because we could not prepare the same number of males as females for the second mating opportunities, there were differences between the numbers of females copulating at the first mating opportunities and the number of females given second mating opportunities.
The fitness components of once-copulated females were measured as follows. A virgin female was put into a well of a 48-well plate in isolation, and maintained for 5 days without beans. The female was then transferred to a 5-mL plastic bottle with a virgin male. Females that copulated within 10 min were transferred to a Petri dish with 20 g adzuki beans and allowed to oviposit until their death. Females that did not copulate within 10 min were excluded from further study. Adult life span, body size, fecundity, and fertility were recorded for all the females. For each mating the male was selected from the same strain as the female. The percentages of the females that copulated during 10-min mating opportunities in the low strain and in the high strain were 87.0 (20/23) and 93% (28/30), respectively. Females of the adzuki bean beetle rarely oviposit without beans (Rönn et al. 2006). We can therefore assume that the ages at which the females started oviposition were not different for once-copulated females and twice-copulated females (5 days after eclosion).
Statistical analysis
The number of hatched eggs and fecundity were analyzed by ANCOVA involving the number of matings, the strain, their interaction, and female body size as a covariate. We analyzed the hatching rate of eggs using generalized linear models (GLM) involving the number of matings, the strain, their interaction, and female body size in the models. The error distribution and the link function were quasi-binomial distribution and logit function, respectively. We analyzed adult life span with the Mann–Whitney U test or, when the variances were significantly unequal, the robust rank-order test (Kasuya 2001). We could not measure the elytra length of two females in the experiment because of a failure in the dissection. These two females were therefore excluded from both ANCOVA and GLM. ANCOVA and the Mann–Whitney U test were performed in JMP (SAS Institute). GLM and the robust rank-order test were performed in R version 2.4.0 (R Development Core Team 2006).
Results
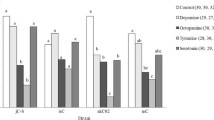
The number of matings had a significant effect on the number of hatched eggs. The increase of the mean number of hatched eggs for the low strain was larger than for the high strain and a significant interaction was detected for number of hatched eggs between strains and the number of matings (Fig. 1a, Table 1).
Comparison of the fitness components of adzuki bean beetle females mated once and mated twice from the low strain (open circles) and the high strain (filled circle). a The mean number of hatched eggs. b The fecundity, or the mean number of eggs laid. c The fertility, or mean hatching rate of eggs. The error bars indicate standard deviations. The sample sizes of the low strain females that mated once and that mated twice are 20 and 26, respectively, and those of the high strain females that mated once and that mated twice are 27 and 24, respectively
The number of matings and strains also had significant effects on fecundity. The increase in fecundity for the low strain was larger than for the high strain and a significant interaction for fecundity between strains and the number of matings was detected (Fig. 1b, Table 2). Alhough the number of matings did not have a significant effect on fertility (the hatching rate of eggs), the increase of fertility in the low strain was larger than in the high strain and a significant interaction for fertility between strains and the number of matings was detected (Fig. 1c, Table 3). The adult life span did not significantly differ between mating treatments for the low strain (Mann–Whitney U test, U = 170.00, P = 0.08; one mating: n = 20, 12.25 ± 1.97 days; two matings: n = 24, 10.96 ± 1.23 days) or for the high strain (robust rank-order test, z = 1.88, P = 0.06; one mating: n = 27, 11.37 ± 2.10 days; two matings: n = 24, 11.96 ± 1.20 days).
Discussion
If the different female mating rates between the strains reflects the different current benefits of polyandry between the strains, the high strain females should gain larger benefits from polyandry than the low strain females. In this study a significant interaction for the number of hatched eggs between the number of matings and strains was detected. This statistical interaction means that the difference between the fitness component of once-copulated females and that of twice-copulated females, i.e. the benefit of polyandry, were different between the two strains. Contrary to the prediction, however, the low strain females enjoyed a larger benefit of polyandry. This suggests that the different female mating rates between the strains would not reflect the different benefits of polyandry, suggesting that the actual mating rates of females would not reflect the current interests of female adzuki bean beetles.
This suggests that female mating rate in each strain would be affected by sexual conflict. Sexual conflict over mating between males and females can lead to mating behavior that is not optimum for one or both of the sexes because of sexually antagonistic co-evolution (Parker 1979; Chapman et al. 2003; Arnqvist and Rowe 2005). In several reviews it has been suggested that more polyandrous species tend to benefit from polyandry, indicating that, on a large scale, the female mating rate for each species may reflect the magnitude of the benefits that the females can gain from polyandry (Ridley 1988; Vahed 1998). It has, however, also been pointed out that, affected by sexual conflict, the actual mating rate of females would not be identical with the optimum mating rate of females on a small scale (Arnqvist and Nilsson 2000). Our study supports this prediction by directly comparing the benefit of polyandry of a low-mating-rate population with that of a high-mating-rate population.
What kind of mechanism causes the difference between the actual female mating rates and the optimum mating rate? In the adzuki bean beetle it has been shown that extracts of male reproductive organs inhibit female mating activity (Takashi Yamane and Takahisa Miyatake: Okayama University, personal communication). Thus, for example, low strain males may transfer more refractory-inducing substances to females than high strain males. It is also possible that all males from both strains transfer the same amounts of ejaculate but that low strain females are more sensitive to the refractory-inducing substances than the high strain females.
Our results also mean that the magnitude of the benefits females gain from polyandry varies substantially among strains of the same species. This point is important, because it has been questioned why there is large variation among the results of studies on the benefits of polyandry even for the same species. That is, some studies report the benefit of polyandry whereas others studies of the same species do not (reviewed by Arnqvist and Nilsson 2000). Our study suggests, however, that the difference between the strains or populations used in the previous studies may, to some extent, explain the variation among the results of studies on the benefits of polyandry in the same species.
There are three problems with this study. First, there is a potential sampling bias in the experiment in which twice-copulated females are compared with once-copulated females (Torres-Vila et al. 2004; Harano et al. 2006). Harano et al. (2006) showed that the body sizes of females accepting re-matings were significantly larger than those of females that refused re-matings and that re-mating treatment actually caused sampling bias in the high strain. In this study we took sampling bias because of female body size into consideration by performing ANCOVA with female body size as a covariate. We could not, however, take into consideration sampling bias because of other unknown factors, for example female activity. We also did not measure the fitness component of females that refused re-matings, which makes it difficult to evaluate whether sampling bias also occurred in the low strain. This problem may somewhat weaken our conclusion.
Second, in this study we covered only a part of possible indirect benefits of polyandry, i.e. the fitness gains of the offspring. The increase in the number of hatched eggs for the low strain females (Fig. 1c; Table 3) may result from indirect benefits that the females gain from polyandry (Tregenza and Wedell 2002). We did not, however, cover other indirect benefits, for example the growth rate or the mating success of the offspring. In the low strain, twice-mated females produce nearly twice as many hatched eggs as the once-mated females. It is, therefore, unlikely that the high strain females acquire larger indirect benefits outweighing this advantage in the low strain. Measurement of the indirect benefits of polyandry in the adzuki bean beetle in future studies will be important, however.
Finally, it is possible low strain females may not be able to gain such a large benefit from polyandry in the natural environment, because factors in natural environment, for example high predation risk during copulation, may not favor polyandry. Little is known of the mating behavior of the adzuki bean beetle in nature. There is insufficient data on selection pressure in the natural environment on polyandry of the low strain and the high strain, respectively. Because the low strain has been bred in captivity under laboratory conditions for a very long period, however, whereas the high strain has not, the results of our study may reflect the lengths of time for which each strain has been cultured rather than the natural environments of each strain (Harano and Miyatake 2005).
This is the first study that directly compared the benefits of polyandry of a low mating-rate population with that of a high mating-rate population. We suggest that the magnitude of the benefits of polyandry is different between the strains and that the different fitness benefits of polyandry between the strains cannot explain the different female mating rate between the strains in the adzuki bean beetle. This suggests that female mating rates of the adzuki bean beetle are affected by sexual conflict.
References
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145-164. doi:10.1006/anbe.2000.1446
Arnqvist G, Rowe L (2005) Sexual conflict. Princeton University Press, New Jersey
Bateman AJ (1948) Intra-sexual selection in Drosophila. Heredity 2:349–368
Birkhead TR, Møller AP (1998) Sperm competition and sexual selection. Academic , San Diego
Chapman T, Arnqvist G, Bangham J, Rowe L (2003) Sexual conflict. Trends Ecol Evol 18:41–47. doi:10.1016/S0169–5347(02)00004–6
Gwynne DT (1997) The evolution of edible ‘sperm sacs’ and other forms of courtship feeding in crickets, katydids and their kin (Orthoptera: Ensifera). In: Choe JC, Crespi BJ (eds) The evolution of mating systems in insects and arachnids. Cambridge University Press, New York, pp 110–129
Harano T, Miyatake T (2005) Heritable variation in polyandry in Callosobruchus chinensis. Anim Behav 70:299–304. doi:10.1016/j.anbehav.2004.10.023
Harano T, Yasui Y, Miyatake T (2006) Direct effects of polyandry on female fitness in Callosobruchus chinensis. Anim Behav 71:539–548. doi:10.1016/j.anbehav.2005.05.017
Harano T, Miyatake T (2006) Interpopulation variation in female re-mating is attributable to female and male effects in Callosobruchus chinensis. J Ethol (in press). doi:10.1007/s10164-006-0204-8
Hosken DJ, Stockley P (2003) Benefits of polyandry: a life history perspective. Evol Biol 33:173–194
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev 75:21–64. doi:10.1017/S0006323199005423
Kasuya E (2001) Mann–Whitney U test when variances are unequal. Anim Behav 61:1247–1249. doi:10.1006/anbe.2001.1691
Miyatake T, Matsumura F (2004) Intra-specific variation in female re-mating in Callosobruchus chinensis and C. maculatus. J Insect Physiol 50:403–408. doi:10.1016/j.jinsphys.2004.02.007
Newcomer SD, Zeh JA, Zeh DW (1999) Genetic benefits enhance the reproductive success of polyandrous females. Proc Natl Acad Sci USA 96:10236–10241
Parker GA (1979) Sexual selection and sexual conflict. In: Blum MS, Blum NA (eds) Sexual selection and reproductive competition in insect. Academic, New York, pp 123–166
R Development Core Team (2006) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ridley M (1988) Mating frequency and fecundity in insects. Biol Rev 63:509–549
Rönn J, Katvala M, Arnqvist G (2006) The costs of mating and egg production in Callosobruchus seed beetles. Anim Behav 72:335–342. doi:10.1016/j.anbehav.2005.10.024
Torres-Vila LM, Rodíguez-Molina MC, Jennions MD (2004) Polyandry and fecundity in the Lepidoptera: can methodological and conceptual approaches bias outcomes? Behav Ecol Sociobiol 55:315–324. doi:10.1007/s00265-003-0712-2
Tregenza T, Wedell N (1998) Benefits of multiple mates in the cricket Gryllus bimaculatus. Evolution 52:1726–1730
Tregenza T, Wedell N (2002) Polyandrous females avoid costs of inbreeding. Nature 415:71–73. doi:10.1038/415071a
Utida S (1941a) Studies on experimental population of the azuki bean weevil, Callosobruchus chinensis (L.). I. Mem Coll Agr, Kyoto Imp Univer 48:1–31
Utida S (1941b) Studies on experimental population of the azuki bean weevil, Callosobruchus chinensis (L.). IV. Mem Coll Agr, Kyoto Imp Univer 51:1–26
Vahed K (1998) The function of nuptial feeding in insects: a review of empirical studies. Biol Rev 73:43–78
Yanagi S, Miyatake T (2003) Costs of mating and egg production in female Callosobruchus chinensis. J Insect Physiol 49:823-827. doi:10.1016/S0022-1910 (03)00119-7
Yasui Y (1997) A “good-sperm” model can explain the evolution of costly multiple mating by females. Am Nat 149:573–584. doi:10.1086/286006
Yasui Y (1998) The ‘genetic benefits’ of female multiple mating reconsidered. Trends Ecol Evol 13:246–250. doi:10.1016/S0169-5347(98)01383–4
Zeh JA, Zeh DW (1996) The evolution of polyandry I: intragenomic conflict and genetic incompatibility. Proc R Soc Lond B 263:1711–1717
Zeh JA, Zeh DW (1997) The evolution of polyandry II: post-copulatory defences against genetic incompatibility. Proc R Soc Lond B 264:69–75. doi:10.1098/rspb.1997.0010
Acknowledgments
Professor Tetsukazu Yahara provided valuable advice and criticism throughout the study. Professor Takahisa Miyatake and Doctor Tomohiro Harano provided laboratory strains of the adzuki bean beetle for this study, and valuable advice. Takashi Kuriwada made comments on the manuscript. This study was in part supported by a Grant-in-Aid of Scientific Research from the Japan Society for the Promotion of Science (Nos. 16370013 and 16370045) to Eiiti Kasuya. We would like to thank our colleagues at the Laboratory of Ecology, Kyushu University for help and encouragement.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sakurai, G., Kasuya, E. Different female mating rates in different populations do not reflect the benefits the females gain from polyandry in the adzuki bean beetle. J Ethol 26, 93–98 (2008). https://doi.org/10.1007/s10164-007-0036-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-007-0036-1