Abstract
The present paper discusses experimental results obtained on several mortars prepared using a commercial type1 OPC cement, a commercial limestone aggregate with maximum particles dimension below 5 mm, superplasticizer and water. Different amounts of a waste silica sand, in form of loose powder, derived from the industrial production of refractory blocks, were added to the above basic components. Several additional samples were also prepared using same basic commercial components added by different amounts of a fine limestone filler. This latter set of compositions was used to prepare samples to be used as reference materials. Waste silica sand and fine limestone filler were used as received from their producer. During preparation, pastes containing silica sand showed better workability compared to all the other blended components. All hydrated materials displayed fair compressive strength and low water absorption after curing, but those containing silica sand showed the best mechanical performances due to their low residual porosity and to the pozzolanic reaction which could enable application for the production of structures in direct contact with water or aggressive environments such as sewers or tanks suitable for the containment of organic substances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silica sand (SS) powders are used as inert insulating medium during the production of refractory electro-fused blocks made by means of electric arc furnaces. These blocks are then used in the manufacture of furnaces for glass production. The industrial process involves: first the melting of the powders mixture containing ZrO2, SiO2 and Al2O3, then molten blend is poured into the moulds and cooled down to room temperature at a very low cooling rate. This sensitive long step requires, as thermal insulator, a silica sand mixture containing 30 wt.% of diatomaceous earth. This latter is added by the refractory industry because of its absorbent properties. When the blocks are at room temperature, they are removed from the moulds and the silica sands are used for further preparations. After certain numbers of production cycles, the exhaust sands must be replaced by new products and the exhaust powders are mainly discarded to landfill; the production of 1 tonne of refractory blocks also implies the production of about 4 tonnes of waste silica sand. However, due to their composition and to the small dimensions of powder particles, exhaust silica sands are classified as harmful toxic product which implies highly expensive disposal in special landfills. In fact, the EU directive 97/96CE of Dec 5th 1997, establishes that when a powder waste contains fibres or coarse particles having size in the range between 50 and 500 µm and, at the same time, contains a total amount of K2O + Na2O + MgO + CaO equal or smaller or than 18 wt.%, it must be considered carcinogenic and requires special landfills for disposal.
In a circular economy context, which possibly avoids the use of landfill disposal of most industrial waste or by-products, the present research aims to propose a possible way for recycling the above-described waste silica sand by the production of mortars. This environmental approach which started several years ago, has led the research world to identify and develop new products which sometimes find application into the construction industry. For example, high-performing mortars or concretes, which imply homogenization of mixtures containing cement, aggregate and water, may be added to other components, such as fly ash, silica fume, ground granulated blast furnace slag (ggbs), glass cullet and others [1,2,3,4,5,6]. A wide literature documents the successful use of fly ash and silica fume as partial cement replacement materials in mortars or concrete mix, in which pozzolanic reaction may improve concrete strength and durability [7,8,9]. On the other hand, glass cullet from different origins could be successfully used as aggregate [10,11,12,13,14,15]. It is as well generally accepted that three main characteristics enable pozzolanic reaction between silica-based additions and cement-based products: i. high silica content; ii. presence of amorphous silica phase and iii. small particles dimensions which, in turn means high specific surface area of the powders.
The use of silica-based components in mortars or concretes production could also cause alkali-silica reaction (ASR) when silica-rich particles react with the alkali in the pore solution of mortars or concretes inducing internal stresses, expansion, and overall affecting materials durability, but could be minimised when the powders particles have small size [2, 7, 9, 14, 16, 17].
The use of industry by-products or waste in mortars and/or concrete production, in partial substitution for aggregate or cementitious materials, depending on their chemical composition and grain size, is therefore generally accepted, provided that the mixture of the starting components is easily homogenised, that pastes have good workability, that the hardened materials display high strength and that the release of eventual hazardous components from the hardened materials (introduced by the waste) does not exceed the established norms.
In the present research, several mortars were produced using a fixed amount of natural limestone aggregate, as it has been often proposed in literature [18, 19], a fixed water-to-cement (w/c) and superplasticizer to cement (s/c) ratios, whereas waste silica sand was added in different proportions thus modifying the global a/c ratio. The addition of superplasticizer was considered necessary to maintain sufficiently low the w/c ratio after the addition of silica sand. In parallel, a series of samples with same ratios as above, were added with a limestone powder having same maximum particles size as the silica sand in order to evaluate the eventual benefits of silica addition to the properties of the resulting hardened mortars. In fact, it is expected that waste silica sand, although fully crystalline, could beneficially affect particle size distribution of the global mortar systems. This, in turn, improves workability of the pastes during materials production and could promote pozzolanic reaction during hardening, but not ASR in hardened products, thus leading to the production of mortars with long-term mechanical performances which open a new way to avoid the need for landfill disposal of this waste. All hardened specimens were characterised by compressive strength and water absorption.
The results obtained from this work represent a fundamental contribution in the context of the construction of large-scale structures involving considerable heat development during the cement hydration phase. These results are of particular importance in the context of the design and construction of structures that require superior resistance to external agents and prolonged durability. These include structures in direct contact with water, such as hydraulic works, dams or bridges, or cast-in-place structures that will involve contact with aggressive environments and extreme conditions once in use. Examples of such structures might involve the construction of sewers, tanks suitable for the containment of organic substances, or offshore platforms intended for the extraction of natural resources. The in-depth knowledge of thermal processes and materials characteristics developed through this research provides a solid basis for optimising construction methods, ensuring the structural safety and reliability of works even under adverse environmental conditions [20].
Experimental details
Starting materials
The starting materials employed in this study were a commercial Type 1 ordinary Portland cement (OPC) and a natural limestone aggregate with maximum particle dimension of 4.76 mm, density of 2.45 g cm−3 and water absorption 0.27% (the measurement was carried out following the ASTM C127 and C128 norms) which were mixed with different proportions of a waste silica sand derived from the production of refractory blocks. A series of samples with addition of a fine limestone powder having same maximum particles dimension as the silica sand was also produced. The Glenium 51 (BASF) was used as a superplasticizer in the preparation of all the specimens. A fixed selected amount of water was added to each starting blend.
The mix proportion design is reported in Table 1 which also displays samples’ symbolic names (used hereafter), water-to-cement (w/c), aggregate to cement (a/c) and superplasticizer to cement (s/c) ratios in all the compositions.
Table 2 shows chemical composition (reported in terms of oxides) and density of cement, silica sand and limestone filler. The chemical analysis was obtained by means of a Spectro Mass 2000 Induced Coupled Plasma (ICP) mass spectrometer whereas densities were determined following the ASTM C127 and C128 standards.
The crystalline phases of as-received silica sand and limestone filler powders were identified by X-ray diffraction (XRD). XRD patterns were recorded by a Philips X’Pert diffractometer (40 kV and 40 mA, Ni-filtered Cu-Kα radiation) using a step size of 0.02° and a counting time of 40 s per angular abscissa in the range of 5–80°. Philips X’Pert High Score software was used for phase identification and semi-quantitative analysis (RIR method) [21].
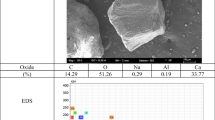
SEM micrographs of the two powders were obtained by a Zeiss EVO40 Scanning Electron Microscope (SEM) coupled with the Energy-Dispersive X-ray Spectroscopy (EDXS).
The particle size distribution (PSD) of silica sand and the limestone filler were determined, in water, after 3 min sonication, by a Horiba LA950 laser scattering PSD analyzer; logarithmic abscissa was used for curves representation. The PSD of the commercial aggregate and those of the mixtures prepared with the addition silica sand and limestone filler obtained using a set of sieves with mesh of 0.238, 0.595, 1.000, 2.380, and 4.760 mm were used as it is suggested by the ASTM C144 official norms.
Mortar samples preparation
For the mixture homogenization and w/c optimization, a 5 L Hobart stirrer conforming to ASTM C305 standards was used. At the conclusion of a preliminary investigation on the reference blend, the optimised amount of water was determined by a modified ASTM C1437 slump test [22, 23]. In this procedure, slurries were poured into a truncated conical mould (top diameter = 70 mm, bottom diameter = 100 mm, height = 60 mm) which was filled up to the top; it was then lifted vertically, the cake was shaken for 5 s, and the diameter of the spread slurry was measured along two perpendicular directions. The slump value was derived from the average of the two measurements. Using this procedure, the pastes are assumed to have sufficient workability when the cake width is 150 (±20) mm. The identified w/c value of the reference blend was 0.45 and this value was used for all the compositions. Slurries were then poured under vibration into cubic (100 × 100 × 100 mm) and/or cylindric (Φ = 30 mm and h = 60 mm) moulds respectively, sealed with a plastic film to ensure mass curing and aged 48 h for a first hydration. Demoulded samples were further cured in water at room temperature for 7, 28, and 90 days. The ageing water was maintained at the constant temperature of 25 °C (±3 °C) and replaced with fresh water every 3 d of curing. After curing, before materials characterisation, samples were dried with a cloth and aged in the atmosphere for 24 h.
Characterisation of hardened materials
Compression tests were performed on cubic specimens (100 × 100 × 100 mm), in accordance with the ASTM C39 standard, using an 810 Material Test System (MTS) with a crosshead speed of 1.5 mm/min. Data were averaged over 3 measurements for each composition.
A modified ASTM C 642 standard was followed to measure water absorption of the hardened cylindric samples. Following this procedure, specimens were dried in an oven at 80 ± 5 °C for 24 h and weighed in air (W1); they were then aged in autoclave at 120 °C and 2 kPa for 2 h using 2 L of water, cooled down to room temperature (in water), dried with a cloth and weighed again (W2). Water absorption was evaluated according to the following equation:
Scanning electron observations of the as-received limestone filler and silica sand were performed by means of a Zeiss EVO40 Scanning Electron Microscope (SEM) coupled with an EDS system.
Results and discussion
Table 2 shows that lime filler contains about 95 wt% CaO with low amounts of MgO and SiO2 whereas the silica sand contains about 95% wt SiO2 together with Fe2O3 (2.9 wt%); other components were detected in very low amounts, whereas heavy metals or other hazardous compounds were not identified at all. The XRD (Fig. 1a and b) investigation revealed the only presence of calcite (PDF 01-086-0174) in the limestone filler, whereas in the SS powder, SiO2 is shared into alpha quartz (PDF 01-083-2465) and alpha cristobalite (PDF 01-076-0938).
Figure 2a and b shows the PSD curves of limestone filler and silica sand. It can be observed that both powders display a trimodal distribution with maximum particles size lower than 1 mm. However, limestone filler has maximum particle concentration at 238 μm (80% of the total volumetric amount) and displays two other small concentration peaks at 0.4 and 5 μm. On the other hand, silica sand shows maximum concentration of particles at around 9 μm (60%) and two smaller concentration peaks at around 80 (20%) and 700 μm (20%) respectively. Therefore, the silica sand has a greater number of fine particles compared to the limestone filler.
Figure 3a and b shows SEM micrographies (2500×) of limestone filler and silica sand powders. Each image shows the presence of facetted, irregular particles which appear to be agglomerated into soft clusters. In addition, Fig. 3b also highlights several fossil relic particles reasonably due to diatomaceous earth.
Figure 4 shows the cumulative representation of particles size distribution obtained with standard sieves acquired on the as-received, commercial natural aggregate. It may be highlighted the very low fraction of particles with size below 149 μm (2.6 wt%), many particles with size between 149 and 1000 μm and a limited amount coarse particles with size greater than 1000 μm.
The granulometric composition above described seems to be far from the ideal one according to Fuller or Bolomey, the amount of small particles being too low and the amount of particles with size between 149 and 1000 μm too high, implying the production of mortar pastes that are neither sufficiently workable nor sufficiently packed and strong hardened materials.
The addition of limestone filler or silica sand modifies the PSD of the original natural aggregate as well as the ratio a/c displayed in Table 1 which starts from 5 of the reference composition and reaches the value of 6.5 of compositions LF3 and SS3. Figure 5a and b shows the PSD trends of the compositions containing 400, 500, and 600 g of each of these powders. However, it is worthwhile to observe that, whilst limestone filler carries into the mixture a great amount of particle with size between 238 and 1000 µm (which is a range of particles already present in great amount into the natural aggregate), silica sand increases the fraction of fine particles (those with size lower than 149 μm) improving workability of the mixture after the addition of water and cement. This apparently speculative statement is however confirmed by Fig. 6 which shows trend of the slump flow versus amount of LF or SS additions of the slurries prepared. In fact, an almost linear decreasing trend with the addition of LF can be observed, conversely SS improves slurries flowability up to the addition of 500 g of powder. However, a greater amount induces a reversal trend. Compositions containing more than 600 g of LF or SS were not prepared due to their extremely poor workability.
The above-described behaviour is reasonably due to the presence of micronic particles which have been introduced into the mixtures by the addition of SS. Literature widely documents the relation between their presence and rheological behaviour of cement-based slurries, highlighting their possible role as fine aggregate as well as supplementary binding material [24,25,26,27]. Particles with size smaller than 75 μm may develop cementitious capability resulting from hydration or pozzolanic reaction. In addition, LF contains small particles. However, the main quantity has size greater than 75 µm with limited effects on rheology behaviour of the resulting cement-based slurries. The reversal trend highlighted on materials containing more than 500 g of SS is reasonably due to the high specific surface area of the fine fraction of silica sand and to the presence of diatomaceous earth with its absorbent properties.
The results obtained from compression strength tests, with relative error bars, displayed as a function of curing time of the compositions containing LF and SS, are showed in Fig. 7a and b which also report the trend of the reference composition. Figure 7a shows that the average compressive strength of LF-containing mortars is 16 MPa after 7 d of curing, 38 after 28 and 40 after 90. This proves that the addition of lime filler improves, but not optimises, the particle size distribution of the starting aggregate.
Figure 7b displays the trend of compressive strength as a function of curing time of mortars manufactured using SS. It may be observed that composition SS1 displays a strength of 22 MPa after 7 days of curing and grows at 57 after 90; SS2 reaches 25 MPa after 7 days and enhances at 78 after 90 days of curing; conversely SS3 shows the same strength of SS2 after 7 days, but longer times leads to materials with lower compressive strength than composition SS2.
Figure 8 shows the trends of strength vs curing time of the blank reference, composition LF2 and SS2 which are respectively the compositions which displayed the best performances of each series of samples. It may be observed that SS2 shows a continuous strength growth and the best overall behaviour. In addition, it has been observed that after the compression tests, all SS-containing samples, although after long curing time in moist environment, showed dried cores thus documenting their low interconnected open porosity.
Table 3 displays the water absorption of materials after 28 and 90 days of curing which look relatively low. It must be observed a significant difference between numbers obtained from samples containing silica sand and those made using limestone filler: the former are lower than the latter in this way highlighting materials with lower open porosity. Additionally, the results obtained on materials aged for 90 days show that samples containing lime filler remain almost constant (in line with the reference composition), whereas those resulted from specimens made with silica sand display a growing trend.
The averaged (3 measurements) values from the compression strength tests of the reference composition after 7, 28, and 90 days are 15, 24, and 26 MPa respectively, whereas water absorption is almost constant at 6.8%. These numbers appear low if compared to those expected from mortars prepared made using a Type 1 cement and are reasonably due to the low amount of cement introduced in all the compositions prepared in the present investigation and to the poor quality of the as-received commercial aggregate, which contains a low amount of fine particles and leads to the production of low quality mortars. The results obtained from the compressive strength tests of mortars containing FS and SS are however higher than data obtained on the reference composition.
The worse strength behaviour of SS3 with respect to SS2 is reasonably due to the worse workability of such composition which leads to a greater air entrapment during homogenization leading to the production of materials with higher water absorption and therefore larger open porosity.
It can also be highlighted that after 28 days of ageing in water, the change in length of all samples is always below the normally accepted value of 0.05% suggested in ASTM C33 and in line with literature data obtained in mortars produced using high silica fine powders [17, 28, 29].
In definitive, the high compressive strength values of SS-containing compositions, compared to reference mortars or LF-containing materials, are reasonably due to their low residual porosity (low water absorption) and to the chemical nature of SS powders which cause pozzolanic reaction because of their high SiO2 content [4, 6, 7, 9, 12,13,14, 30,31,32]. The particles size distribution of SS powders improves pastes workability limiting air entrapment and therefore favours the production of materials with low residual porosity. The non-negligible porosity reduction between 28 and 90 days observed on silica sand containing samples, but not on the reference composition nor on materials made using fine lime filler, is reasonably due to the reactive fraction of silica sand and Ca(OH)2 from the hydration process of the cement components to form additional hydrated silicate giving rise to the pozzolanic phenomenon reducing materials porosity [2, 4, 6, 7, 24, 33,34,35,36,37,38,39,40,41,42]. Moreover, after casting, during hydration, the low cement/aggregate ratio leads to the production of materials with an almost absence of expansion and microfracturing.
XRD analysis of the hydrated samples, acquired after 90 days of curing, did not reveal the presence major quantity of hydrated phases in SS-containing specimens with respect to reference or LF-containing compositions probably due to their amorphous or cryptocrystalline nature [43, 44] therefore they are not reported in the presence paper.
The better mechanical behaviour of SS-containing materials with respect to the other compositions prepared in the present research may be therefore due to their chemical composition, which affects materials hydration and to their particles size distribution which favours better workability of the relative mortars’ pastes.
Conclusions
In the present research, the production of stable cement mortars was carried out using a commercial type 1 OPC, a commercial natural aggregate, superplasticizer and water. Production and characterisation of such hydrated reference composition were compared to products that were added with a lime filler and/or a waste silica sand derived from the industrial production of refractory bricks. Mortars were produced using fixed water-to-cement, cement-to-aggregate and cement-to-superplasticizer ratios, whereas lime filler and waste silica sand were added in different proportions.
The following important conclusions were derived from the study:
-
1.
The addition of lime filler and silica sand optimises particles size distribution of the starting mixtures of components due to the low cement content used for all production and to the little amount of fine particles in the starting natural aggregate although silica sand is the product that develops the best behaviour;
-
2.
The low cement-to-aggregate ratio used for preparations limits expansion and micro-fracturing during hydration;
-
3.
The addition of silica sand improves workability of the reference mortars pastes as well as the addition of lime filler up to an upper quantity limit determined by the fixed amount of water used for all the preparations;
-
4.
Lime filler and silica sand containing hydrated materials displayed fair strength in compression tests, but those containing silica sand displayed the best overall behaviour after 7, 28, and 90 days of curing in water due to their low water absorption (low residual porosity) and to the pozzolanic activity caused by the reactive fraction of silica sand.
The results obtained in the present research show that waste silica sand, due to the fine powders particles and to its specific chemical composition, could be easily recycled into the production of cement mortars contributing to improve the performances of materials prepared using not optimised particles size distribution. Such products could be used for the production of structures in direct contact with water or aggressive environments such as sewers or tanks suitable for the containment of organic substances.
References
Wei MS, Huang KH (2001) Recycling and reuse of industrial waste in Taiwan. Waste Manage 21:93–97
Shi C, Qian J (2000) High performance cementing materials from industrial slag-A review. Resour Conserv Recycl 29:195–207
ACI Committee 232 (2001) Use of fly ash in concrete (ACI 232.2R-96), ACI manual of concrete practice, part 1. American Concrete Institute, Farmington Hills
ACI Committee 232 (2000) Use of raw or processed natural pozzolans in concrete (ACI 232.1R-00). American Concrete Institute, Farmington Hills
ACI Committee 233 (2001) Ground, granulated blast furnace slag as a cementitious constituent in concrete (ACI 233R-95), ACI manual of concrete practice, part 1. American Concrete Institute, Farmington Hills
ACI Committee 234 (2001) Guide for use of silica fume in concrete (ACI 234R-96), ACI manual of concrete practice, part 1. American Concrete Institute, Farmington Hills
Mehta PK, Gjorv OE (1982) Properties of Portland cement concrete containing fly ash and condensed silica fume. Cem Concr Res 12:587–595
Helmuth RA (1987) Fly ash in cement and concrete. PCA, Skokie
Cong X, Gong S, Darwin D, McCabe SL (1992) Role of silica fume in compressive strength of cement paste, mortar and concrete. ACI Mater J 89(4):375–387
Harrison WH (1974) Synthetic aggregate sources and resources. Concrete 8(11):41–44
Johnston CD (1974) Waste glass as coarse aggregate for concrete. J Test Eval 2(5):344–350
Meyer C, Baxter S (1997) Use of recycled glass for concrete masonry Blocks. NYSERDA Report, pp 15–97
Polley C, Cramer SM, Cruz RV (1998) Potential for using waste glass in Portland cement concrete. J Mater Civil Eng ASCE 10(4):210–219
Shao Y, Lefort T, Moras S, Rodriguez D (2000) Studies on concrete containing ground waste glass. Cem Concr Res 30(1):91–100
Shayan A, Xu A (2004) Value-added utilization of waste glass in concrete. Cem Concr Res 34:81–89
Reindl J (1998) Report by recycling manager. Department of Public Works, Madison, Dane County
Park SB, Lee BC (2004) Studies on expansion properties in mortar containing waste glass and fibers. Cem Concr Res 34:1145–1152
Pèra J, Husson S, Guillot B (1999) Influence of finely ground limestone on cement hydration. Cement Concrete Res 21:99–105
Qasrawi H, Shalabi F, Asi I (2009) Use of low CaO unprocessed steel slag in concrete as fine aggregate. Constr Build Mat 23:1118–1125
Coppola L (2007) Concretum. McGraw-Hill ed, Milan
Jenkins R, Snyder R (1996) Introduction to X-ray powder diffractometry. Wiley ed, New York.
Wang Q, Li L, Wu CP, Sui ZT (2009) Research on adaptability of slag-based geopolymer with superplasticizer. Key Eng Mater 405:129–134
Nematollahi B, Sanjayan J (2014) Effect of different superplasticizers and activator combinations on workability and strength of fly ash based geopolymer. Mater Design 57:667–672
Chen CH, Huang R, Wu JK, Yang CC (2006) Waste E-glass particles used in cementitious mixtures. Cement Concrete Res 36:449–456
Saccani A, Bignozzi MC (2010) ASR expansion behaviour of recycled glass fine aggregates in concrete. Cement Concrete Res 40:531–536
Ducman V, Mladenovic A, Suput JS (2002) Lightweight aggregate based on waste glass and its alkali–silica reactivity. Cement Concrete Res 32:223–226
Husem M (2003) The effects of bond strengths between lightweight and ordinary aggregate-mortar, aggregate-cement paste on the mechanical properties of concrete. Mater Sci Eng A 363:152–158
Shi C, Wu Y, Riefler C, Wang H (2005) Characteristics and pozzolanic reactivity of glass powders. Cem Concr Res 35:987–993
Topcu IB, Canbaz M (2004) Properties of concrete containing waste glass. Cem Concr Res 34:267–327
Shayan A, Xu A (2006) Performance of powder as pozzolanic materials in concrete: A filed trail on concrete slabs. Cem Concr Res 36:457–468
Byars EA, Morales-Hernandez B, Zhu HY (2004) Waste glass as concrete aggregate and pozzolan. Concrete 38(1):41–44
Corinaldesi V, Gnappi G, Moriconi G, Montenero A (2005) Reuse of ground waste glass as aggregate for mortars. Waste Manage 25(2):197–201
Collepardi M (1991) Scienza e Tecnologia del Calcestruzzo, 3rd edn. Hoepli ed, Milan
Tangpagasita J, Cheerarotb R, Jaturapitakkula C, Kiattikomola K (2005) Packing effect and pozzolanic reaction of fly ash in mortar. Cement Concrete Res 35:1145–1151. https://doi.org/10.1016/j.cemconres.2004.09.030
Glodman A, Bentur A (1993) The influence of microfillers on enhancement of concrete strength. Cement Concrete Res 23(4):962–972
Isaia GC, Gastaldini ALG, Morases R (2003) Physical and pozzolanic action of mineral additions on the mechanical strength of high-performance concrete. Cement Concrete Comp 25(1):69–76
Erdogdu K, Turker P (1998) Effects of fly ash particle size on strength of Portland cement fly ash mortars. Cement Concrete Res 28(9):1217–1222
Mora EP, Paya J, Monzo J (1993) Influence of different sized fractions of a fly ash on workability of mortars. Cement Concrete Res 23(4):917–924
Bigas JP, Gallias JL (2002) Effect of fine mineral additions on granular packing of cement mixtures. Mag Concrete Res 54(3):155–164
Paya J, Monzo J, Borrachero MV, Peris E, Amahjour F (2000) Mechanical treatments of fly ashes: Part IV. Strength development of ground fly ash-cement mortars cured at different temperatures. Cement Concrete Res 30(4):543–551
Paya J, Monzo J, Borrachero MV, Peris E, Gonzalez Lopez E (1997) Mechanical treatments of fly ashes: Part III. Studies on strength development of ground fly ashes (GFA)-cement mortars. Cement Concrete Res 27(9):1365–1377
Ranganath RV, Sharma RC, Krishnamoorthy S (1995) Influence of fineness and silica content of fly ashes on their strength development with respect to age. ACI-SP 153:355–366
Hewlett PC (1998) Lea’s chemistry of cement and concrete, 4th edn. Arnorld ed, London
Taylor NFW (1997) Cement chemistry, 2nd edn. Thomas Telford ed, London
Funding
Open access funding provided by Università degli Studi di Udine within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Furlani, E., Zanocco, M., Tubaro, E. et al. Waste silica sand as a possible pozzolanic filler to produce cement mortars: experimental investigation. J Mater Cycles Waste Manag 26, 1795–1803 (2024). https://doi.org/10.1007/s10163-024-01939-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-024-01939-1