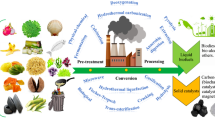
Abstract
Hydrothermal Liquefaction (HTL) is a promising thermochemical treatment suitable for the valorization of a wide variety of organic wastes and wastewaters. Many research studies have demonstrated the suitability of HTL to produce bio-oil from biomass wastes, but few works have focused on the application of HTL to industrial wastes and wastewaters. The objective and novelty of this work are the study of HTL suitability over a specific selection of industrial residues that present notable drawbacks when treated by conventional waste management methods, including technical problems or high costs. Most of the wastes presented poor results from the technical or energetical point of view. However, liquid surfactant wastewaters were successfully treated by HTL at 300ºC and 100 bar in a 300 mL stirred batch reactor, producing a crude oil yield of around 16%, with a High Heating Value (HHV) of up to 30 MJ/kg. The composition and quality of the crude oils obtained have also been determined and compared against conventional biomass fuels.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world is currently facing an energy crisis, and for the year 2040, energy demand has been predicted to increase by up to 35% [1]. Thermochemical processes (mainly pyrolysis, combustion, liquefaction and gasification) have been gaining a considerable attention in recent years because of their potential use to valorize not only municipal and biomass waste, but also industrial wastes.
HTL can be applied to the treatment of water containing organics at moderate temperatures (between 280 and 350 ºC), while pressure level varies according to temperature so that water is maintained in its liquid state (100–200 bar) [2]. Under these conditions, the organic molecules break at their weakest bonds and subsequently multiple recombination of their fragments take place, which results in a final product that contains a wide variety of organic substances (linear alcohols, ketones, cyclics, furfurals, phenols, …) and that is considered to resemble the characteristics of crude oil (also known as bio-oil when the product comes from the treatment of biomass). The main objective sought by applying HTL is to obtain high yields of this crude oil, since this product exhibits a High Heating Value (HHV) making it suitable to be used as a sustainable alternative to fossil fuels.
HTL is a really interesting technology for the treatment of biomass. Several reviews [2,3,4,5,6,7] and studies [8,9,10,11,12,13,14] can be found in the literature on the application of HTL to model compounds (saccharides, furans, glycerol, fatty acids, amino acids, lignin and so on) as well as on complex waste or wastewaters (woods, sawdust, sewage sludge, algae, garbage, manures, agri-food wastewaters, etc.). In most cases, their outcome is rather positive in terms of bio-oil yields. While Canché-Escamilla et al. [15] studied pyrolysis of the henequen plant obtaining 40% bio-oil yield and 20.54 MJ/kg HHV at 700 ºC, Giroto et al. [16] reviewed the pyrolysis and HTL of Spent Coffee Grounds concluding that HTL is most convenient due the moist nature of this waste (that can reach up to 85% of wet mass), confirming that with a low temperature of 275 °C, it is possible to obtain 47.3% bio-oil yield with 31.0 MJ/kg HHV. Regarding the treatment of industrial wastes, Hu et al. [17] compared pyrolysis and HTL of vehicle tires. For the HTL, a 45% crude oil yield was obtained at 400 ºC with a HHV of 42.02 MJ/kg. HTL could be even more promising if it could be successfully applied to the large amounts of aqueous wastes that is rated by numerous industries. As an example, just the surfactant industry generates around 13 million of metric tons of waste per year globally [18] and a large part of such waste is disposed on lands or into water systems. Linear alkylbenzene sulfonates (LAS) and alkyl phenol ethoxylates (APE) are the most common surfactants [19] and they produce toxic metabolites (i.e. nonylphenols) when they are broken down under aerobic conditions, so biological treatments are not often applied to industrial surfactant wastes.
To our knowledge, no industrial surfactants have been treated with HTL up to date. Yang et al. [20] studied the HTL including surfactants, but only as an additive in the pre-treatment of sewage sludge with subcritical water, obtaining a 15.5% increase in the calorific value of the product. Table 1 gathers some of the most representative studies that can be found in the literature and that support the suitability of this technology for the treatment of organic wastes.
The bio-oil yields that have been obtained and their quality, in terms of combustion power, depend not only on the raw material, but also, on a number of operating variables such as temperature, residue concentration, pH, catalyst, reaction time, pressure or the addition of different gases into the reactor. Several authors have studied the effect of those variables on the specific biomasses used to produce bio-oils [2, 3]. The following HTL variables and their corresponding effect on the resulting yields have been considered when determining the most appropriate operating conditions to be used in this work:
Temperature
Temperature is clearly one of the most influential HTL operating parameter. When the temperature reaches above the level required to break the chemical chains, the depolymerization of the biomass takes place, causing the concentration of free radicals and enabling the re-polymerization into bio-oil. In most cases, as can be seen in Fig. 1, the optimum temperature level is around 300 °C, which is in agreement with a considerable number of authors [9, 23,24,25,26,27,28].
Residue concentration
Given that water generally acts as a solvent that improves the stability and solubility of the fragmented components, a large amount of water is adequate for the production of crude oil and gases [2] and low dry-mass/solvent ratios (from 0.02 to 0.16) result in reduced leftovers and gas yields [9, 25].
pH
In general, acidic conditions result in greater yields than those obtained from alkaline media. Typically, the crude oil contains considerable amounts of furfurals [5] compounds (mainly 5-Hydroxymethylfurfural) if they are produced in acid or neutral media, and carboxylic acids (C2–C5) in alkaline media [29]. Homogeneous catalysts in the form of alkali salts are more frequently used and according to their efficiency in a decreasing order, these are K2CO3 > KOH > Na2CO3 > NaOH [6].
Reaction time
Short residence times are better for an effective partial degradation of the compounds in the feed. Furthermore, a zero reaction time, i.e., stopping the heating once the target temperature has been reached, has revealed to give place to the best yields from the HTL of cellulose [7].
It appears that the hydrothermal conversion routes into liquid fuels are especially favorable for biomass wastes and particularly for biopolymers (cellulose, hemicellulose, lignin), proteins and lipids. Nevertheless, there is no reason why it should not be possible to find certain organic molecules of industrial origin that could be converted into liquid fuel by HTL. The objective of this paper is to evaluate the possibility to obtain crude oil from HTL of industrial residues. As a novelty, in the present work, HTL has been applied to a selection of seven different industrial residues, including solid and liquid surfactants that are handled by a Spanish waste management company. All those wastes were selected, because their disposal by conventional methods is not satisfactory and they fulfill the requirements to be considered suitable for HTL in terms of water solubility, moisture and organic content (see “Industrial waste, equipment and experimental procedures”). Among them, surfactant wastes should be considered particularly interesting because of the large amounts of wastes that they represent. Different operating conditions (temperature, concentration, pH and reaction time) have been studied using a 300 mL stirred batch reactor. The composition and quality of the liquid products has also been determined and compared against conventional biomass fuels by using their atomic oxygen/carbon and hydrogen/carbon ratios.
Materials and methods
Industrial waste, equipment and experimental procedures
Selection of industrial waste
All the industrial wastes and wastewaters were supplied by VERINSUR Corp., a Spanish waste management company (https://www.verinsur.com/). Since HTL uses water as the reaction medium and transforms organic material into crude oil, the selection of the wastes was based in the following general priorities:
-
Aqueous feed with > 85% moisture content, and > 5% organic matter content. Solid wastes are suitable if they are water-soluble and the required aqueous feed can be prepared.
-
Pumpable liquid, emulsion or viscous liquid of a density that may carry some suspended solids or particles as long as they are < 10 mm or, even preferably, filtered.
-
In order to avoid corrosion, ideal wastes should also be free from salts, with a pH between 4 and 10 and no halogenated compounds.
The exact composition of the wastes selected is unknown, but the main properties of the raw surfactant wastes were provided by VERINSUR Corp. and are shown in Table 2. It is important to point out that the above selection policy is based on general priorities, but it was not possible to find any residue in VERINSUR that fulfills all the priorities. All the wastes tested, their origin and some of their most relevant characteristics are summarized below:
Solid surfactant waste
Waste matter from a surfactant powder manufacturing company: 116,780 mgC/kg of dissolved organic carbon (DOC in leachate), significant presence of sulphates (4420 mg/kg), chlorides (464 mg/kg), and also some nitrates (145 mg/kg).
Liquid surfactant wastewaters
Dense liquid disposed from the cleaning of a production line in a liquid surfactant manufacturing company, concentrated by reverse osmosis. Two different lots of this wastewater, with samples received on different dates, have been used to verify the results. The compositions of the two lots are presented in Table 2, and their average composition is as follows: 38,255 mgC/L DOC, high chloride content (1864 mg/L) and the HHV of a dry sample from this wastewater reached 19.06 MJ/kg.
Glycerine wastes
Dense and viscous liquid (distilled glycerine) discarded from the cleaning of tanks in a cosmetic manufacturing company: 311,970 mgC/L DOC and 2625 mg/L sulphates. Due to the high viscosity of glycerine, it was necessary to dilute it at a rate of 1/5 in deionized water to allow the rotation of the reactor´s stirrer. The HHV of the raw waste was determined as 15.39 MJ/kg.
Alkali wastewaters
Aqueous wastewater discarded from the cleaning of tanks in a drug manufacturing company (alkali boiler bottom waters): 87,050 mgC/L DOC, high presence of chlorides (12,248 mg/L) and sulphates (16,878 mg/L). This raw wastewater was diluted at a rate of 1/5 in deionized water to keep DOC at a similar level as that of the other wastes tested.
Industrial process wastewaters I
Diluted aqueous stream resulting from the cleaning of varied items from the surfactant industry: 18,050 mgC/L DOC, high presence of chlorides (65,727 mg/L), sulphates (21,424 mg/L), and metals, such as boron (29 mg/L) or zinc (92 mg/L).
Industrial process wastewaters II
Process waters from the cleaning of varied items from the surfactant industry: 16,000 mgC/L DOC, considerable presence of chlorides (51,438 mg/L), sulphates (23,453 mg/L) and also some iron (9 mg/L).
Cleaning wastes
Liquid waste from the cleaning of household chemical product tanks: 49,850 mgC/L DOC. Quite high content of chlorides (67,859 mg/L) and sulphates (21,155 mg/L), as well as boron (49 mg/L) and zinc (13 mg/L).
As above mentioned, more specific data are available regarding the two surfactant wastes tested: I) Solid Surfactant Waste and II) Liquid Surfactant Wastewaters.
Equipment
All the experiments were carried out using a bench scale batch reactor (Autoclave Engineers) of 300 mL volume and fitted with an internal stirring unit (Fig. 2). The reactor body was made of 316 stainless steel and had 286 cm3 functional volume. The heating system to reach and maintain the reaction temperature comprised 3 elements: A 1.2 kW cylindrical electric heater around the reactor with capacity to raise the temperature up to 400ºC. The PID controller (ICP, model TC21), with the capacity to maintaining a ± 2ºC constant temperature level. A k-type thermocouple built into the reactor and connected to the PID controller. The reactor includes a gas inlet and a liquid sampler. A pressure gauge indicates the pressure inside the reactor within the range from 0 to 550 bar. Finally, the stirring unit inside the reactor was a “0.7502 Magnedrive” mechanical agitator with a belt driven head and a magnetic driven propeller.
Experimental procedure
Based on the effects of variables described in the introduction, the following conditions have been selected as the starting point in this work, that is, temperature around 300 ºC, reaction time < 15 min, 0.11 to 0.34 dry-mass/water ratio and neutral pH.
150 mL samples (liquid and/or solid samples diluted in deionized water) were placed inside the reactor, which was subsequently closed and purged with a flow of nitrogen gas for 15 min. Once the air is purged, more nitrogen gas was injected into the reactor from a 50 L, 200 bar tank until the pressure reached around 27 bar at ambient temperature. The pressure level for this specific reactor was established so that the entire reaction would take place in liquid phase (calculated for 150 mL) when the temperature reached up to 300ºC, which meant a pressure between 90 and 100 bar. After setting each experimental reaction temperature the heater was turned on, and the stirring system is set at 440 rpm, which has been demonstrated to ensure the homogenization of the feed inside the reactor. Most of the experiments reached 300ºC and at that moment, the furnace was turned off and removed to allow the reactor to cool down by submerging it into tap water at room temperature. This procedure is identified as zero reaction time and, as it was mentioned in the introduction, it is, according to the literature, the optimal procedure to obtain a higher yield of crude oil. After the reactor had cooled down to room temperature, the gases were extracted and saved into 1 L SKC Tedlar Sample Bags for further analysis. Then, the reactor was depressurized and opened in order to extract its liquid content into test tubes to determine its volume prior to analysis.
Sample pre-treatment
Since the experiments intend to put to the test the suitability of HTL to process residues, the samples were prepared through different procedures according to their nature to avoid altering their pH and other characteristics, while making sure that the reactor’s feed fulfilled certain requirements, such as solid/water ratio and initial COD. In the case of Liquid Surfactant Wastewaters (that will be referred as liquid surfactant from this point) samples, the waste was generally diluted at 1/3 in deionized water, although some undiluted samples were also tested. Furthermore, to determine the effect resulting from the initial pH on the final product, one of the experiments was run using a sample whose pH had been lowered from 6.1 to 2.1 (using 11.3 mL of 5 M ortho-phosphoric acid over a 150 mL 1/3 dilution of the wastewater), while another run was conducted using a basic pH sample which had been added 18.7 mL of 1 M sodium hydroxide to increase its pH from 6.1 up to 8.9, or even after being added Na2CO3 (1% of the feed weight) to reach a pH of 9.9. The solid surfactant waste was diluted at around 1/3 and 2/3 (waste weight/water weight) before being sampled. Finally, to determine the effect from combining liquid and solid surfactant wastes, a mixed feed formed by 1/3 (solid surfactant weight/liquid surfactant weight) was also tested.
Sample post-treatment: crude oil separation
After completing each experimental run and opening the reactor, the liquid product was collected and its volume was measured. In addition, to remove the product that could remain on the inner wall of the reactor as well as on the stirrer and the liquid sampling line these were washed first with deionized water and then with acetone, both of which were also collected. The effluent was poured into a separatory funnel together with the washing water and the acetone that had been used to wash down the reactor. More acetone was added to fully wash down the crude oil. The funnel was subjected to 3 cycles consisting of 2 min agitation and 2 min settling. The content of the separatory funnel was filtered using vacuum and glass fiber filters (Hahnemühle GF52 047); the solids that were trapped on the filter were identified as “solid product”. The filtered liquid was subjected to evaporation in a vacuum rotary evaporator at 80ºC to remove its acetone and water content. The yield (wt%) was calculated based on the weight of the final product (crude oil) compared against the weight of the raw wastewater (in the case of liquids) or the weight of the solids (in the case of solid waste) used to prepare the feed. The operation procedure and analysis implemented have been considered suitable and reproducible, since the experiments carried out in duplicate revealed a 15.2% crude oil yield and 32.47 MJ/kg HHV with 0.3 and 0.56 standard deviation, respectively.
Analytical results
Since industrial wastes contain a vast array of different compounds, chemical oxygen demand (COD) and total organic carbon (TOC) were determined for each one of the wastes and taken as the reference to prepare feeds with similar initial organic content. The composition of the gas phase generated was also determined.
-
Chemical Oxygen Demand (COD): The analysis was carried out according to the dichromate standard method (APHA 2005)[30]. The samples were not filtered for analysis.
-
Total Organic Carbon (TOC): This parameter is representative of the amount of organic matter present in water or wastewaters. A SHIMDZU TOC-5050A total organic carbon analyzer was used and the standard combustion-infrared method (APHA 2005) was followed [30]. The samples were not filtered for analysis.
-
Higher Heating Value (HHV): This is an essential parameter to determine the viability of the crudes obtained and, hence, whether they are suitable to be used as a source of energy. It was determined by means of an isoperibolic Parr 6400 Calorimeter oxygen pump set.
An optimal HTL process should result both in the maximum crude yield and the maximum HHV (or in the best balance between those two). In order to obtain a combined measurement of both parameters (crude yield and HHV) as a single one, the overall energy produced per feed mass was calculated as follows:
-
Characterization of the gaseous effluent: This analysis measures the evolution of the gases that are produced as a result of the hydrothermal liquefaction of the feed. A Hewlett Packard HP 6890 PLUS gas chromatograph was used.
-
Ultimate analysis of the crude oil and solids obtained: “The ultimate analysis of the samples allows to determine the mass percentage of carbon, hydrogen, nitrogen and sulphur. As the analysis did not measure the content of some of the atoms that may be present in the samples (such as P, Na or Cl), the oxygen content was calculated as the difference with the total content, once the amount of ashes has been subtracted. This type of analysis was used to characterize the liquid and solid HTL products. The analyses were performed in a LECO CHNS-932 ultimate analyzer.
Experimental results
In the first stage of the work, the suitability of seven industrial residues to produce crude oil by HTL was tested. Among these residues a mixture of solid and liquid surfactant was included. All the residues were treated under similar operating conditions, which were chosen as the most suitable ones according to the literature regarding the HTL treatment of biomass wastes, i.e., moderate temperature (300ºC), low waste/water ratio (maximum 0.34 in the case of liquid surfactants), short reaction time (from zero to 15 min) and no catalyst (due to the high costs that it would represent at an industrial scale). The results from this first set of experiments are shown in Table 3.
Table 4 shows the set of experiments conducted to determine the influence from both the reaction time and the reaction medium pH on the hydrothermal liquefaction of liquid surfactant Table 5.
Table 6 shows the results of ultimate analysis of the solid products.
An additional set of experiments intended to verify the suitability of the process was carried out using a new lot of the liquid surfactant received several months after completing the first set of experiments (lot 2). The influence of both reaction temperature and feed concentration were determined and shown in Table 7.
Discussion
All the tested industrial wastewaters could be treated by HTL under stable and safe conditions and a processed product could be obtained. However, most of them produced poor results in terms of amount of crude yield and HHV, or even presented severe technical problems regarding their treatment by HTL, even at a laboratory scale. Some of the treated wastes and wastewaters produced large amounts of sticky solids, impurities and poor quality or even non-combustible oils. These have been marked at “non suitable for HTL” in Table 3, since according to the results from the tests, it can be presumed that their treatment at industrial scale would present important disadvantage or hardly any interest regarding energy production. In the following paragraph, the main reasons why some of the selected wastes were discarded for HTL are explained briefly.
The experiments on Alkali wastewater and Industrial Process Wastewater I, resulted in a rather poor outcome, as despite their acceptable crude oil yields (15.3 and 21.6%, respectively), their HHV was extremely low (< 2.10 MJ/kg in both cases). Industrial Process Wastewater II and Cleaning waste provided the worst results. Not only they produced very low yields (2.3% and 8.7%, respectively), but none of them combusted at the calorimeter test. The hydrothermal liquefaction of Glycerine at 300 ºC and using zero reaction time resulted in an apparent crude oil that represented 93% of the feed mass and with just 14.36 MJ/kg HHV, which is 6.69% lower than that of the raw material at 15.39 MJ/kg. It seems evident, therefore, that the HHV of glycerine waste cannot be improved by hydrothermal liquefaction under the tested conditions. To try to improve those results, another experimental run was conducted using 15 min as the reaction time, but an HHV increment of just 0.8% was obtained over untreated Glycerine. Therefore, HTL has not been proven to be a suitable treatment to transform glycerine wastes under the conditions studied.
In the case of solid surfactants, a first test was carried out by dissolving 100 g of solid surfactant waste into 92 g of deionized water to obtain 150 mL of a feed dilution for its hydrothermal liquefaction at 300ºC and zero reaction time (i.e., the oven was turned off once the target temperature had been reached). A black liquid substance was obtained, but a large amount of a soft solid substance was generated that got stuck on the stirrer blades and reactor walls; also, a rock solid substance got stuck at the bottom of the reactor and mechanical means had to be employed to break it into pieces so that it could be removed. In order to minimize the generation of those undesired solids, a second test was conducted using a feed at a lower solid/water ratio, i.e., 50 g of solid surfactant waste dissolved into 121.1 g of deionized water. The problems that had been observed before were reduced and the liquefaction of solids was actually enhanced. It is important to point out that when the black liquid product was filtered, before the evaporation to obtain the final crude, more than 5 g of solids were retained. As can be seen in Table 3, the yields obtained ranged from 17.5 up to 22.1%, but no liquid crude was obtained in any of these experiments where solid surfactant had been used, and the resulting solid crude did not burn when conducting the calorimetry test.
Despite all those wastes were initially considered suitable for the HTL screening based on the requirements shown in “Selection of industrial waste”, after the first set of experiments they were discarded for HTL and no more insight will be shown. On the other hand, Liquid Surfactant Wastewaters were successfully treated by HTL, as can be seen from the data in Table 3. Not only because in this first screening it produced acceptable crude oil yields (16.9%) and high HHV (26.99 MJ/kg), but also because no sticky solids were formed and pH remained around neutrality. For these reasons, the rest of the experiments focused on the HTL of liquid surfactant.
In the following paragraphs, the effect of reaction time, pH, residue concentration, and temperature have been evaluated with respect to the treatment of liquid surfactant with the aim of maximizing crude yields that also exhibit the highest possible HHV. As already mentioned in “Analytical results”, in order to evaluate both parameters (crude yield and HHV) as a single one, the overall energy per feed mass (MJ/kgfeed) will be used for a standardized comparison.
Effect of using different reaction time
Once the necessary time for the organic matter to become crude had elapsed, any additional residence time inside the reactor may lead to a decrease in crude oil yield. This would be explained by a greater formation of gases and/or solids and a lesser amount of organics remaining in the liquid phase.
As can be seen in Table 4, when the reaction time was increased from zero to 15 min the yield went up from 17 to 29%, while, on the contrary, the HHV of the crude oil dropped from 26.99 down to 22.73 MJ/kg. All in all, the overall energy generation increased by 30% (from 1.55 to 2.20 MJ/kg feed). It can, therefore, be seen that a longer reaction time has improved both the yield and the overall energy. According to expectations, a larger amount of solids were filtered from the product when a longer reaction time (15 min) was used, which represents an increments of 11.9% over the amount of solids obtained when the experiment was run using zero reaction time. Most authors recommend short reaction times for HTL of biomass [21, 31, 32]. In the case of HTL of surfactants, an economic study would be required to determine the pros and cons associated to different reaction times and the profitability of the procedure at industrial scale, given that the costs associated to the 15 min heating time must be taken into consideration,
pH influence on the final product
In this work, most experiments were carried out with a near neutral pH. However, in order to verify the consequences of an acid or basic pH medium on the processing of the liquid surfactant, two additional experiments under specific pH conditions were conducted; acidic (2.1) and basic conditions (8.9 and 9.9). Based on the studies shown in the introduction, the alkalis NaOH and Na2CO3 were selected for the basic pH experiments because of their low cost. Given that 6.1 was the pH of the regular liquid surfactant after its dilution in deionized water at 1/3, the first experiment was done at this pH around neutral. Afterward, the pH was lowered down to around 2 by adding phosphoric acid in order to carry out an experiment under acidic conditions, while NaOH or Na2CO3 were added to run the experiments in a basic pH around 9.
The experiments with an initial acidic medium produced only slightly higher crude oil yields (18.7%) and HHV (27.77 MJ/kg) compared to those of the reference experiment (initial pH around 6). As it was found by other authors [6, 24], when NaOH is added, the HTL yield increased considerably, achieving a 25.6% in the case of liquid surfactants. However, the crude oil obtained was a gelatinous substance of a considerably lower HHV (15.24 MJ/kg). Regarding the amount of filtered solids, no relevant differences were found, with 0.18 g of solids in both experiments.
As shown in Table 4, the addition of Na2CO3 allowed the operating temperature to be reduced by 25 ºC and still a yield of 35.6% was obtained, which is the highest value registered in this work. Since HHV also reached a good value (26.91 MJ/kg), the 35.6% yield implied that the overall energy was also one of the highest of all experiments (3.19 MJ/kg feed). The amount of filtered solids was also one of the largest (0.42 g) of all the experiments, since it nearly doubled the amounts obtained from the rest of them.
It is important to point out that the use of an initial acidic or alkaline pH feed produces effluents with extreme pHs, 1.7 and 10.6, respectively. According to the yields that have been obtained, adding 1wt% of Na2CO3 seems a rather promising and profitable approach to produce crude oil by HTL.
Effects of feed concentration (ratios)
As it was described in the Materials and methods section, two different lots of liquid surfactant wastewaters have been used for the experiments. The first lot with a DOC concentration of 21,950 mgC/L was received in February, six months before the second one, which presented 54,560 mgC/L DOC concentration and was received in September of the same year. In both cases a 1/3 dilution of the raw residue in deionized water was prepared, and although the DOC concentration was 2.5 times higher in lot 2, similar crude oils yields were obtained from both of them (around 15–17%). This could be explained by the fact that in both cases the samples contained large amounts of water at a dry-mass to water ratio lower than 0.05, so that a moderate increment in feed concentration did not affect the yields. However, the HHV of the product obtained from lot 2 (32.47 MJ/kg) was higher than the HHV obtained from lot 1 (26.99 MJ/kg), which could be attributed to differences in the lots’ compositions. In fact, the reason to test two different lots is the variations in the composition that generally takes place in industrial wastes.
In order to delve a bit more into the effect of waste concentration, lot 2 feed was tested once more without any dilution, so that it could be compared against the feed that had been diluted at 1/3 in deionized water. As can be seen in Table 7, the yield obtained in both cases was quite similar (around 15% of the total feed). This could be explained by the high content of water in the residue (the raw surfactant wastewater already contained a high percentage of water, over 85%), with a 0.13 “dry-mass/water” ratio, which is within the range suitable for an HTL treatment (0.02–0.16) Therefore, the addition of a bigger amount of water does not seem to improve the reaction, like occurs in the case of solid biomass [2]. On the other hand, the HHV of the product obtained from the undiluted feed was also similar (around 32 MJ/kg) to the value obtained from the diluted wastewater feed from the same lot. It is, nevertheless, important to point out that the overall energy of the product obtained from the undiluted liquid surfactant was considerably higher. In fact, it was the highest one of all the experiments conducted in this study at 5.12 MJ/kgfeed. As expected, more solids were retained by the filters from the undiluted sample than from the 1/3 diluted one, with 0.45 g and 0.18 g, respectively.
From an economic point of view, higher concentrations allow to reduce the volume of the reaction media that must be heated to reach HTL conditions. It seems rather logical to think that lower dilutions of the raw wastes would be more convenient for industrial scale systems, since smaller feeds would reduce pumping, heating and reactor volume requirements to obtain similar yields with higher overall energy generating capacity.
Effect of using different temperatures
Although 300ºC had already been established as an adequate temperature for the HTL process, two more tests were performed at lower (290ºC) and higher temperature (310 ºC) to verify whether the operating conditions could be moderately changed without any noticeable reduction or even an improvement of the yield or the HHV of the crude oil. Table 7 and Fig. 3 show the results from such test.
The total yield slightly decreased from 15.3 at 300 to 14.5% at 290 ºC, and no drastic drop in terms of HHV (32.47 MJ/kg at 300 ºC vs. 31.60 MJ/kg at 290 ºC) was registered. Even though the reduction in the process temperature would favor some energy saving during the heating step, the overall energy fell by up to 6.8%. On the other hand, a temperature of 310ºC did not seem to significantly improve the yield (+ 0.68%), while HHV decreased by 8.1%. On the other hand, a similarly lower overall energy to that obtained from the experiment run at 290ºC confirmed that 300ºC continued to be the best temperature for the HTL of liquid surfactants. This results are coherent with other biomass experiments made by other authors [9, 23,24,25,26,27,28] that were presented in Table 1.
Effect of mixing solid and liquid surfactants
It has been established in previous sections that HTL of solid surfactant wastes presented operational problems due to the formation of large amounts of rocky and sticky solids. Since the treatment of solid surfactant wastes would be very interesting from an environmental point of view, the possibility of processing them together with liquid surfactants in low proportion combinations has been tested. Consequently, in order to determine the effect of combining both types of surfactants, a mixture of 50 g of solid surfactant together with 117 g of liquid surfactant was loaded into the reactor. The first positive consequence was a quite lower solid content in the effluent (five times less). The results are shown in Table 3. The crude oil yield was really high (37%), but it was in solid phase and with a poor HHV (16.16 MJ/kg).
The treatment of solid and liquid surfactant mixtures present some pros and cons. As advantage, two different wastes can be treated in the same process, which would allow different initial mixture ratios and getting a gate fee. However, as disadvantages, larger amounts of solids are obtained in comparison to the HTL of liquid surfactants on their own, which makes the filtering stage rather cumbersome; a solid crude with a lower HHV is obtained and a large amount of ashes is generated (> 40% of its weight) by the combustion of the crude.
From the environmental point of view, a treatment process that could be applied to a combination of both types of wastes would be rather desirable. Unfortunately, from a technical point of view and if a source of power is to be obtained, the hydrothermal liquefaction of liquid surfactants on their own seems to be more feasible and effective.
Characteristics of the crude oils obtained
A practical way to classify the crude oils obtained in the previous sections and compare them against other better known fuels (hydrocarbons, bio-fuels, solid fuels, etc.) consists in determining their atomic oxygen/carbon and hydrogen/carbon ratios and presenting them in a van Krevelen graph. This procedure was followed by Poudel et al. to compare an assortment of fuels obtained against biomass and other more conventional fuels [33, 34]. According to this method, the most efficient fuels would be located within the area defined by high H/C and low O/C ratios, i.e., the top left area in Fig. 4 (where ethane, propane, methane, etc. are located).
Table 5 shows the ultimate analysis of the main crude oils obtained. The crude oils obtained by the HTL of the surfactant wastes are located in a reasonably good H/C and O/C area, i.e. they are in most cases within the dashed line area that includes most fuels obtained from biomass. It is important to point out that the O/C ratios include some error, since the ultimate analysis did not measure the content of some of the atoms that may be present in the crude oil (such as P, Na or Cl). The oxygen content was calculated as the difference with the total content, once the amount of ashes has been subtracted. Ashes were only measured for the best crude oil sample, being approximately 4.3%. Considering that all the crude oils part from the same feed and the content of salts and metals remains in the ashes, the same value has been considered similar for all samples (Table 7).
As an example, the atomic ratios of sample III (crude from solid surfactant) are H/C = 1.86, which is similar that of biomass fuels, and O/C = 1.73, which is a very high value, beyond the limits of this graph and, therefore, has not been included. In this case, the result from the ultimate analysis indicating 63.7% oxygen content is clearly excessive for organic compounds and, therefore, a lower content should be expected.
Table 8 shows the parameter acceptance requirements of a fuel to be considered as an alternative fuel to be used in the cement industry for comparison against the values corresponding to the crude oils obtained. The crude oil obtained by HTL from raw liquid surfactant, that is, without any dilution, can be used to establish this comparison, as it is considered as the most successful outcome among all the tests that have been carried out in this work. The comparison reveals that this crude oil is a promising alternative to traditional fossil fuels.
Regarding the solids retained on the filters, their ultimate analysis revealed that they cannot be considered as char, due to their low to moderate carbon content. The solid product generated in the experiments burns with a HHV of 19.83 MJ/kg; thus, it could be a usable by-product. However, the amount of solids produced is rather small at between 0.2 and 2% by weight.
On the contrary, the gases that are generally obtained through HTL reactions under subcritical temperatures are not normally of any use as fuel. For example, from the liquid surfactant, just 0.17 g of gases were generated, of which 85.46% were CO2, 3.96% O2 and 10.58% CO. Other more interesting gases from the power generation point of view such as hydrogen or methane have not been found, being 100 ppm the detection limit. Figure 5 shows the mass balance, composition and main results corresponding to the experiment that achieved the best results through the HTL of liquid surfactants. In all cases, the mass balances are very close to 100%.
The lack of operating problems in the experiment corresponding to Fig. 5, as well as the rather promising results regarding yield, HHV and overall energy make us think that HTL could be successfully applied to certain industrial surfactant wastes.
Conclusions
This work has demonstrated that HTL can be a suitable process to produce crude oils from some limited industrial wastes. It has also been proved that the selection of the waste based on its apparent suitability for the HTL process does not lead to a successful outcome of the process. In fact, most of the tested industrial wastewaters gave rather unsatisfactory results (low yield, poor HHV) or presented a series of serious technical problems when subjected to HTL, such as large production of solids and other impurities or poor quality of the final crude oils.
The best results in this work were achieved by applying HTL to liquid surfactant, where no technical problems arose and a crude oil was obtained with good yields (up to 16.9%) and HHV (up to 32.47 MJ/kg). The yield obtained is lower than those obtained for HTL of biomass, ranging from 17 to 75% (Table 1) and the HHV obtained is similar to those obtained for biomass. Taking into account that the treatment of industrial wastes by HTL may open a field of applications, the results obtained in this work can be considered quite acceptable. Besides, the crude oils obtained in this work were generally of an acceptable quality, with high hydrogen and carbon contents, while oxygen and sulphur were detected in mild proportions. Attending to their high H/C and low O/C atomic ratios, the fuels generated from the treatment of wastes by HTL could be grouped together with other conventional biomass fuels. The selected conditions for HTL of liquid surfactant residues were a temperature of 300ºC under 100 bar with reaction times lower than 15 min, at a concentration from 0.10 to 0.35 (dry-mass/water) and without the addition of any additives, which produced a liquid crude oil that fulfills the acceptance requirements in terms of % ashes, HHV and sulphur content.
There is no doubt that further studies will be required before HTL can be applied to the production of fuels from industrial wastes at a larger scale. Nevertheless, and according to the rather promising results from this work, the actual possibility of producing alternative fuels and, at the same time, tackling a series of major environmental problems, make of HTL an attractive technology that should be seriously considered.
References
Carstens H. The World Needs More Energy. ExpronewsCom 2019:1. https://geoexpro.com/2019/05/the-world-needs-more-energy/ (accessed May 18, 2021).
Akhtar J, Amin NAS (2011) A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew Sustain Energy Rev 15:1615–1624. https://doi.org/10.1016/j.rser.2010.11.054
Toor SS, Rosendahl L, Rudolf A (2011) Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy 36:2328–2342. https://doi.org/10.1016/j.energy.2011.03.013
Tekin K, Karagöz S, Bektaş S (2014) A review of hydrothermal biomass processing. Renew Sustain Energy Rev 40:673–687. https://doi.org/10.1016/j.rser.2014.07.216
Gollakota ARK, Kishore N, Gu S (2018) A review on hydrothermal liquefaction of biomass. Renew Sustain Energy Rev 81:1378–1392. https://doi.org/10.1016/j.rser.2017.05.178
Kumar M, Olajire Oyedun A, Kumar A (2018) A review on the current status of various hydrothermal technologies on biomass feedstock. Renew Sustain Energy Rev 81:1742–1770. https://doi.org/10.1016/j.rser.2017.05.270
Yang J, He Q, Yang L (2019) A review on hydrothermal co-liquefaction of biomass. Appl Energy 250:926–945. https://doi.org/10.1016/j.apenergy.2019.05.033
Minowa T, Zhen F, Ogi T (1998) Cellulose decomposition in hot-compressed water with alkali or nickel catalyst. J Supercrit Fluids 13:253–259. https://doi.org/10.1016/S0896-8446(98)00059-X
Yin S, Dolan R, Harris M, Tan Z (2010) Subcritical hydrothermal liquefaction of cattle manure to bio-oil: Effects of conversion parameters on bio-oil yield and characterization of bio-oil. Bioresour Technol 101:3657–3664. https://doi.org/10.1016/j.biortech.2009.12.058
Çağlar A, Demirbaş A (2001) Conversion of cotton cocoon shell to liquid products by supercritical fluid extraction and low pressure pyrolysis in the presence of alkalis. Energy Convers Manag 42:1095–1104. https://doi.org/10.1016/S0196-8904(00)00122-9
Demirbaş A (2000) Effect of lignin content on aqueous liquefaction products of biomass. Energy Convers Manag 41:1601–1607. https://doi.org/10.1016/S0196-8904(00)00013-3
Castro A, Anzola A, Cagua I, Rodríguez LI, Agamez Y, Hernández O et al (2008) Potassium carbonate effect on biocrude functionalgroups evolving from lignocellulosic biomass undergoinghydrothermal conversion close to the critical pointof water. Rev Colomb Química 37:243–251
Itoh S, Suzuki A, Nakamura T, Yokoyama S (1994) Production of heavy oil from sewage sludge by direct thermochemical liquefaction. Desalination 98:127–133. https://doi.org/10.1016/0011-9164(94)00137-5
Suzuki A, Nakamura T, Yokoyama S, Ogi T, Koguch K (1988) Conversion of sewage sludge to heavy oil by direct thermochemical liquefaction. J Chem Eng Japan 21:288–293
Canché-Escamilla G, Guin-Aguillón L, Duarte-Aranda S, Barahona-Pérez F (2022) Characterization of bio-oil and biochar obtained by pyrolysis at high temperatures from the lignocellulosic biomass of the henequen plant. J Mater Cycles Waste Manag 24:751–762. https://doi.org/10.1007/s10163-022-01361-5
Girotto F, Pivato A, Cossu R, Nkeng GE, Lavagnolo MC (2018) The broad spectrum of possibilities for spent coffee grounds valorisation. J Mater Cycles Waste Manag 20:695–701. https://doi.org/10.1007/s10163-017-0621-5
Hu Y, Attia M, Tsabet E, Mohaddespour A, Munir MT, Farag S (2021) Valorization of waste tire by pyrolysis and hydrothermal liquefaction: a mini-review. J Mater Cycles Waste Manag 23:1737–1750. https://doi.org/10.1007/s10163-021-01252-1
Chacha S (2018) Development of an analytical method by visible spectrophotometry to determine anionic detergents in clean and wastewater. Universidad central del Ecuador
Scott MJ, Jones MN (2000) The biodegradation of surfactants in the environment. Biochim Biophys Acta - Biomembr 1508:235–251. https://doi.org/10.1016/S0304-4157(00)00013-7
Yang T, Liu X, Li R, Li B, Kai X (2019) Hydrothermal liquefaction of sewage sludge to produce bio-oil: Effect of co-pretreatment with subcritical water and mixed surfactants. J Supercrit Fluids 144:28–38. https://doi.org/10.1016/j.supflu.2018.10.005
Islam MN, Park JH (2018) A short review on hydrothermal liquefaction of livestock manure and a chance for Korea to advance swine manure to bio-oil technology. J Mater Cycles Waste Manag 20:1–9. https://doi.org/10.1007/s10163-016-0566-0
Singh R, Prakash A, Balagurumurthy B, Singh R, Saran S, Bhaskar T (2015) Hydrothermal liquefaction of agricultural and forest biomass residue: comparative study. J Mater Cycles Waste Manag 17:442–452. https://doi.org/10.1007/s10163-014-0277-3
Zhang B, von Keitz M, Valentas K (2009) Thermochemical liquefaction of high-diversity grassland perennials. J Anal Appl Pyrolysis 84:18–24. https://doi.org/10.1016/j.jaap.2008.09.005
Sugano M, Takagi H, Hirano K, Mashimo K (2008) Hydrothermal liquefaction of plantation biomass with two kinds of wastewater from paper industry. J Mater Sci 43:2476–2486. https://doi.org/10.1007/s10853-007-2106-8
Qu Y, Wei X, Zhong C (2003) Experimental study on the direct liquefaction of Cunninghamia lanceolata in water. Energy 28:597–606. https://doi.org/10.1016/S0360-5442(02)00178-0
Shuping Z, Yulong W, Mingde Y, Kaleem I, Chun L, Tong J (2010) Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy 35:5406–5411. https://doi.org/10.1016/j.energy.2010.07.013
Karagöz S, Bhaskar T, Muto A, Sakata Y, Uddin MA (2004) Low-temperature hydrothermal treatment of biomass: Effect of reaction parameters on products and boiling point distributions. Energy Fuels 18:234–241. https://doi.org/10.1021/ef030133g
Zhou D, Zhang L, Zhang S, Fu H, Chen J (2010) Hydrothermal liquefaction of macroalgae enteromorpha prolifera to bio-oil. Energy Fuels 24:4054–4061. https://doi.org/10.1021/ef100151h
Yin S, Tan Z (2012) Hydrothermal liquefaction of cellulose to bio-oil under acidic, neutral and alkaline conditions. Appl Energy 92:234–239. https://doi.org/10.1016/j.apenergy.2011.10.041
Andrew D 2005 Eaton; American Public Health Association. In: Andrew D (eds) American Water Works Association Water Environment Federation Standard Methods for the Examination of Water and Wastewater. Washington, DC
Obeid R, Lewis DM, Smith N, Hall T, van Eyk P (2020) Reaction kinetics and characterisation of species in renewable crude from hydrothermal liquefaction of monomers to represent organic fractions of biomass feedstocks. Chem Eng J 389:124397. https://doi.org/10.1016/j.cej.2020.124397
Elliott DC, Biller P, Ross AB, Schmidt AJ, Jones SB (2015) Hydrothermal liquefaction of biomass: developments from batch to continuous process. Bioresour Technol 178:147–156. https://doi.org/10.1016/j.biortech.2014.09.132
Castro Vega AA, Rodríguez Varela LI, Velásquez D, de J. (2007) Subcritical hydrothermal conversion of organic wastes and biomass Reaction pathways. Inge Investig 27:41–50
Poudel J, Karki S, Oh SC (2018) Valorization of waste wood as a solid fuel by torrefaction. Energies. https://doi.org/10.3390/en11071641
Acknowledgements
This work has been co-financed by the 2014-2020 ERDF Operational Programme and by the Department of Economy, Knowledge, Business and University of the Regional Government of Andalusia. Project reference: FEDER-UCA18-108297. Acknowledgements to Servicios centrales of University of Cádiz for the expertise advice in the ultimate analysis of crude-oil and solids. We would also like to acknowledge the management company Verinsur for the financial support, the technical information and the wastes supplied for this study.
Funding
Funding for open access publishing: Universidad de Cádiz/CBUA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mascarell, J.J., Ruiz-Jorge, F.J., Abelleira-Pereira, J.M. et al. Production of crude oil from industrial wastes and wastewaters by hydrothermal liquefaction. J Mater Cycles Waste Manag 25, 3476–3489 (2023). https://doi.org/10.1007/s10163-023-01771-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01771-z