Abstract
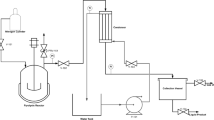
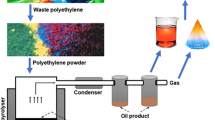
Thermocol which is made of polystyrene is used for different purposes including insulation and packaging. After use, it is thrown away as waste, and hence contaminates the environment. Therefore, the present study is focused on conversion of thermocol into fuel oil using nickel oxide as catalyst. Thermogravimetry and a pyrolysis chamber were used to pyrolyze thermocol waste both in presence and absence of catalyst. In the pyrolysis chamber, the highest oil yield was obtained at 370 °C, 5% catalyst, and 70 min reaction time. GC–MS was performed of the obtained oil and it was observed that the quantity of some fuel-range hydrocarbons such as ethyl benzene had increased to 35%. Additionally, TG analysis of the sample with/without catalyst was carried out at different heating rates and the resultant information was used for calculating kinetic parameters applying Coats Redfern (CR) and Ozawa Flynn Wall (OFW) models. The use of catalyst reduced the activation energy and enhanced the quality of oil. The oil produced from thermocol waste in the presence of a catalyst was compared to diesel, gasoline, and kerosene oil and found as a viable fossil fuel alternative.






Similar content being viewed by others
References
Cole M et al (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62(12):2588–2597
Bockhorn H, Hornung A, Hornung U (1998) Stepwise pyrolysis for raw material recovery from plastic waste. J Anal Appl Pyrol 46(1):1–13
Aguado J, Serrano D, Escola J (2008) Fuels from waste plastics by thermal and catalytic processes: a review. Ind Eng Chem Res 47(21):7982–7992
Basha MH, Sulaiman SA, Uemura Y (2020) Co-gasification of palm kernel shell and polystyrene plastic: effect of different operating conditions. J Energy Inst 93(3):1045–1052
Supramono D, Listiono EV, Nasikin M (2019) Effect of hydrogen gas pressure on biofuel characteristics in hydrogenation reaction of non-oxygenated fraction of bio-oil. In: AIP conference proceedings. AIP Publishing LLC
Paço A et al (2019) Biotechnological tools for the effective management of plastics in the environment. Crit Rev Environ Sci Technol 49(5):410–441
Ali MF, Siddiqui MN (2005) Thermal and catalytic decomposition behavior of PVC mixed plastic waste with petroleum residue. J Anal Appl Pyrol 74(1–2):282–289
Filip M et al (2013) Investigation of thermal and catalytic degradation of polystyrene waste into styrene monomer over natural volcanic tuff and Florisil catalysts. Open Chem 11(5):725–735
Hussain Z et al (2020) White cement and burnt brick powder catalyzed pyrolysis of waste polystyrene for production of liquid and gaseous fuels. Asia-Pac J Chem Eng 15(1):e2391
Chumbhale VR et al (2004) Catalytic degradation of expandable polystyrene waste (EPSW) over mordenite and modified mordenites. J Mol Catal A: Chem 222(1–2):133–141
Pakiya Pradeep A, Gowthaman S (2022) Combustion and emission characteristics of diesel engine fuelled with waste plastic oil—a review. Int J Ambient Energy 43(1):1269–1287
Pradeep AP, Gowthaman S (2022) Extraction of transportation grade fuels from waste LDPE packaging polymers using catalytic pyrolysis. Fuel 323:124416
Lee T et al (2020) Catalytic pyrolysis of polystyrene over steel slag under CO2 environment. J Hazard Mater 395:122576
Wei T-T et al (2010) Chemical recycling of post-consumer polymer waste over fluidizing cracking catalysts for producing chemicals and hydrocarbon fuels. Resour Conserv Recycl 54(11):952–961
Filip MR, Pop A, Rusu T (2011) GS-MS and FTIR studies of the liquid products obtained by thermal and catalytic degradation of polystyrene waste. Stud Univ Babes Bolyai Chem 56(4):1
Parasuram B, Karthikeyan S, Sundram S (2013) Catalytic pyrolysis of polystyrene waste using bentonite as a catalyst. J Environ Nanotechnol 2:97–100
Nisar J et al (2020) Pyrolysis of polystyrene waste for recovery of combustible hydrocarbons using copper oxide as catalyst. Waste Manag Res 38(11):1269–1277
Park K-B et al (2020) Two-stage pyrolysis of polystyrene: pyrolysis oil as a source of fuels or benzene, toluene, ethylbenzene, and xylenes. Appl Energy 259:114240
Özsin G, Pütün AE (2018) Co-pyrolytic behaviors of biomass and polystyrene: kinetics, thermodynamics and evolved gas analysis. Korean J Chem Eng 35(2):428–437
Nisar J et al (2022) Pyrolysis of juice-squeezed grapefruit waste: effect of nickel oxide on kinetics and bio-oil yield. Int J Environ Sci Technol 2022:1–12
Ali G et al (2020) Thermo-catalytic decomposition of polystyrene waste: Comparative analysis using different kinetic models. Waste Manag Res 38(2):202–212
Nisar J et al (2020) Pyrolysis of waste tire rubber: a comparative kinetic study using different models. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, p 1–11
Nisar J et al (2021) Cobalt-doped molecular sieve for efficient degradation of polypropylene into fuel oil: kinetics and fuel properties of the oil. Chem Eng Res Des 2021:1
Vyazovkin S et al (2011) ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520(1):1–19
Nisar J et al (2021) Production of fuel oil and combustible gases from pyrolysis of polystyrene waste: kinetics and thermodynamics interpretation. Environ Technol Innov 24:101996
Nisar J et al (2022) Conversion of polypropylene waste into value-added products: a greener approach. Molecules 27(9):3015
Nisar J et al (2022) Production of fuel oil from decomposition of polypropylene over Cu–Co modified molecular sieve-based catalyst. Chem Eng Res Des 2022:1
Flynn JH, Wall LA (1966) General treatment of the thermogravimetry of polymers. J Res Nat Bur Stand 70(6):487–523
Coats A, Redfern J-P (1965) Kinetic parameters from thermogravimetric data. II. J Polym Sci Part B Polym Lett 3(11):917–920
Coats AW, Redfern J (1964) Kinetic parameters from thermogravimetric data. Nature 201(4914):68–69
Ali G et al (2021) Production of liquid fuel from polystyrene waste: process optimization and characterization of pyrolyzates. In: Combustion science and technology, pp 1–14
Nisar J et al (2020) Pyrolysis of almond shells waste: effect of zinc oxide on kinetics and product distribution. In: Biomass conversion and biorefinery, p 1–13
Ramesan M et al (2019) Nickel oxide@ polyindole/phenothiazine blend nanocomposites: preparation, characterization, thermal, electrical properties and gas sensing applications. J Mater Sci Mater Electron 30(14):13719–13728
Qiao H et al (2009) Preparation and characterization of NiO nanoparticles by anodic arc plasma method. J Nanomater 2009:1
Mateos D et al (2019) Synthesis of high purity nickel oxide by a modified sol–gel method. Ceram Int 45(9):11403–11407
Hajakbari F (2020) Characterization of nanocrystalline nickel oxide thin films prepared at different thermal oxidation temperatures. J Nanostruct Chem 10(1):97–103
Liu X et al (2018) Engineering mesoporous NiO with enriched electrophilic Ni3+ and O− toward efficient oxygen evolution. Catalysts 8(8):310
Xu X et al (2017) Mesoporous high surface area NiO synthesized with soft templates: remarkable for catalytic CH4 deep oxidation. Mol Catal 441:81–91
Zhang Z et al (1995) Chemical recycling of waste polystyrene into styrene over solid acids and bases. Ind Eng Chem Res 34(12):4514–4519
Nisar J et al (2019) Pyrolysis of expanded waste polystyrene: influence of nickel-doped copper oxide on kinetics, thermodynamics, and product distribution. Energy Fuels 33(12):12666–12678
Lisa G et al (2003) Thermal behaviour of polystyrene, polysulfone and their substituted derivatives. Polym Degrad Stab 82(1):73–79
Brems A et al (2011) Thermogravimetric pyrolysis of waste polyethylene–terephthalate and polystyrene: a critical assessment of kinetics modelling. Resour Conserv Recycl 55(8):772–781
Miandad R et al (2017) Effect of plastic waste types on pyrolysis liquid oil. Int Biodeterior Biodegradation 119:239–252
Nisar J et al (2019) Fuel production from waste polystyrene via pyrolysis: kinetics and products distribution. Waste Manag 88:236–247
Verma A, Sharma S, Pramanik H (2021) Pyrolysis of waste expanded polystyrene and reduction of styrene via in-situ multiphase pyrolysis of product oil for the production of fuel range hydrocarbons. Waste Manag 120:330–339
Fan S et al (2022) Microwave-assisted pyrolysis of polystyrene for aviation oil production. J Anal Appl Pyrol 162:105425
Amjad UES et al (2021) Diesel and gasoline like fuel production with minimum styrene content from catalytic pyrolysis of polystyrene. Environ Progress Sustain Energy 40(2):e13493
ASTM (1979) Annual book of ASTM standard part 23, American Society of Testing Materials, Philadelphia, PA. Annual Book of ASTM Standard Part 23, American Society of Testing Materials, Philadelphia, PA
IP (1993) Standards for petroleum and its products, part I, Institute of Petroleum. Standards for Petroleum and Its Products, Part I, Institute of Petroleum London
Acknowledgements
Higher Education Commission, Pakistan is acknowledged for Grant No. 20-1491.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Ethical approval
This article is an original work not submitted to/published elsewhere in any form or part. Proper acknowledgements to other works are given and the results are presented clearly, honestly, and without fabrication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nisar, J., e Hina, G., Ali, G. et al. Conversion of thermocol waste into fuel oil over nickel oxide: kinetics and fuel properties of the oil. J Mater Cycles Waste Manag 25, 2996–3004 (2023). https://doi.org/10.1007/s10163-023-01736-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01736-2