Abstract
In this work, biogas was synthesized from malt enriched-craft beer bagasse with the objective to generate clean energy. Thus, a kinetic model based on thermodynamic parameters was proposed to represent the process with coefficient determination (R2) of 0.82. A bench-top biodigester of 2.0 × 10–3 m3 was built in glass, and equipped with sensors to measure pressure, temperature, and methane concentration. The inoculum selected for the anaerobic digestion was the granular sludge, and malt bagasse was used as substrate. Data were fitted to a pseudo-first-order model for the formation of methane gas using the Arrehnius equation as basis. For the simulations of biogas production, the Aspen Plus™ software was used. Results from 23 factorial design experiments evidenced that equipment was efficient, and the craft beer bagasse showed great biogas production, with nearly 95% of methane yield. The temperature was the variable that showed most influence in the process. Moreover, the system has a potential for the generation of 10.1 kWh of clean energy. Kinetic constant rate for methane production was 5.42 × 10–7 s−1 and activation energy 8.25 kJ mol−1. A statistical analysis using a math software was performed and evidenced that the temperature played a major role in the biomethane conversion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beer production has an important strategic position in the world food industry, since its global consumption has totaled 187.58 billion liters per year according to data from 2016 [1], with almost 92% being produced in forty main producing countries, being the US, China and Brazil the leading countries of this process, with most of the breweries in the world [2]. In this regard, craft beer industries have been growing over the past years, despite their small piece in the market share. Even in the middle of covid-19 pandemic crisis in 2020, when craft brewing industry’s continual run of positive growth rates has come to a halt, it showed profitable results, as reported by Brewers Association [3].
During beer production, the residue generated on a larger scale is malt bagasse. In general, for every 100 kg of processed grains, 125 to 130 kg of wet bagasse are generated, with around 80 to 85% moisture, which corresponds to around 14 to 20 kg of bagasse for each hectoliter of beer produced. Normally, this residue is destined for animal feed, burning or composting [4]. Knowing the possibility of better destinations (2G ethanol production, anaerobic fermentation for biogas production, production of value-added compounds, etc.) it is of fundamental importance to develop viable techniques for the best use of this residue.
One of the possible ways of applying the waste from the brewing process is the production of biogas. The biogas produced from bagasse from beer production can be employed to generate energy in the industries, contributing to energy integration. Therefore, it can be a feasible alternative to obtain savings in process costs. However, the literature regarding the use of solid wastes from craft beer industries in anaerobic digestion (AD) is rather scarce when compared to other sources, such as food wastes [5, 6], bagasse from plantation [7], cow, cattle, swine and chicken manure [8, 9], and sludges from sewage and effluent treatment plants [10]. This practice becomes interesting in face of the continuous growing of environmental, social and corporate governance (ESG) practices; one of them, the reducing of the consumption of fossil fuels (coal, natural gas, oil and its derivatives) [11]. This energy matrix represents 82% of the primary source of energy in most countries [12]. Fossil fuels present themselves as a critical panorama when relating such sources to environmental and socio-political problems when considered the existing reserves and the longevity of these resources. Given these concerns, renewable fuels, such as biomass (e.g., sugarcane, palms and malt bagasse), have been highlighted as a way of escaping dependence on fossil fuels and oil derivative products [13,14,15].
One of the most widespread processes to produce biogas from biomass source is the AD [10, 16]. This technology has great potential for application to concentrated or diluted organic effluents, mainly due to the low production of sludge (5 to 10 times lower than what occurs in aerobic processes) [17]. Anaerobic digesters stand out as equipment with low economic and energy costs, low solids production, tolerance to high organic loads and possibility of operation with long solids retention times. Because it is soluble and easily fermented, malt bagasse can be well applied to biodigesters, as it degrades into volatile fatty acids (VFAs) very quickly, favoring biological treatment, especially anaerobes [16].
Currently, there are several tools used to study different processes, and kinetic modeling as well as phenomenological simulation are the ones that have a great contribution to the prediction of results. Using these instruments, it is possible to understand the phenomena that occur during AD process and employ them for scale-up and optimization of operating conditions. Therefore, the main objective of this work is to highlight the effectiveness of the use of malt bagasse from craft beer industry to produce biogas. Another aim was to perform a process simulation and proposing a kinetic model for the process based on Arrhenius equation, which is another innovation approach of this work.
Methodology
Acquisition and storage of malt residue and inoculum
The malt residue was obtained from Grunhs Bier™ craft brewery located in the city of Garanhuns, Pernambuco State, Brazil. The inoculum used was industrial sludge (granulated anaerobic sludge), obtained from wastewater treatment plant of Musashi, located in Igarassu, Pernambuco State, Brazil. Both malt residue and sludge were collected and stored in sealed containers and kept under refrigeration at temperature 292 K.
Characterization of malt residue and inoculum
Malt residue (substrate) and anaerobic sludge (inoculum) were characterized through the analysis of volatile solids (VS), the samples were placed in a muffle EDG model Thermo-3000; pH measurements were performed in a Digimed DM23 pH-meter. Electrical conductivity of the samples was acquired by conductimetric method through the DM23 pH-meter. Finally, elemental analysis (Carbon, nitrogen, sulfur and hydrogen) was carried out in a Carlo-Erba-Instruments model EA 1110. For the substrate, volatile acidity, alkalinity, chemical oxygen demand (COD, in mg O2/L) and biochemical oxygen demand (BOD, in mg O2/L) analyzes were performed with the objective to investigate the conversion of organic matter into biogas. Three analyzes were carried out: one for the sludge used in the biodigester; one for the malt bagasse before biodigestion; and for the malt bagasse after the experiment of the biodigestion process.
Experimental bench reactor
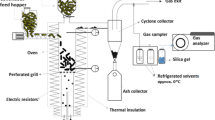
A 2.0 × 10–3 m3 bench-top biodigester built in glass was assembled and equipped with a drain and three side inserts. The experimental apparatus, and the software used are schematized in Fig. 1a. Instruments installed in the biodigester were pressure, temperature and methane sensors, model DS18B20 (Maxim integrated), in addition to a supervisory data control. In addition to the biodigester design, the project had specific software that was capable of instantly recording (in-line) data, at predetermined intervals of time, with a graphical interface to ease the understanding of the AD behavior.
An anaerobic granular sludge was used as inoculum and malt residue as substrate, as depicted in the process flow chart (Fig. 2). The digester was inoculated with about 5.0 × 10–5 m3 of granular sludge and 0.005 kg of substrate, both of which were previously characterized. The initial pH was 7.0 for all treatments, this value is within the favorable range for biogas generation. The temperatures used were 293 K, 298 K and 303 K, which correspond to the working temperatures of the equipment. Each monitoring (in-line) took place between 7 and 15 days with the data being automatically collected by the software and the analyses performed weekly. In the experiments were considered the variables temperature, reaction time and amount of substrate (biomass ratio, in kg m−3). The identification of the biomethane content (CH4) was carried out through a specific gas sensor, the sensor also identifies the formation of CO2, the sensor datasheet is attached. Finally, a kinetic study was carried out with the data modeled in a math software to obtain parameters for the kinetic model.
Theory and calculations
Kinetic modeling
For the elaboration of the kinetic model, it was considered the evolution of biogas production as a function of time. The adjustment of the kinetic model to the results was performed with the help of MathLab™ software, creating a specific program for the proposed model.
For this work, an adjustment to a pseudo-first order (PFO) kinetic model was suggested, since it was necessary to consider the formation profile only with CH4, disregarding the CO2 production phase. In this way, a concentration profile that resembles a first-order system was obtained. As the Aspen Plus™ software was used for the later stage of the work to run the simulations, it has in its reactional package bank the use of a first-order kinetic model. To fit the simulator parameters, the preference was given to fit the experimental data to the PFO kinetic model.
In this work, only the formation step of the biomethane component (CH4) in the biogas production process was studied, disregarding the steps of formation of other gases. In this sense, the model proposed in this work is semi-empirical, considering analogy with chemical kinetics and linear dependence of the process factors. The elaboration of a kinetic model was possible using a first-order differential equation solution, adapted from the Arrhenius model [18, 19] considering a pseudo-first-order reaction and they are presented by Eqs. 1–5.
where CA is substrate concentration (in mg L−1); CA0 is the initial substrate concentration (in mg L−1); k is the PFO kinetic constant (in min−1); t = reaction time (in min); c is the constant of integration (dimensionless).
The product concentration is given by Eqs. 6–10:
where Cg is the concentration of CH4; q is the stoichiometric coefficient (dimensionless); α is a mathematical representation of the term of proportionality [19].
The Arrhenius constant defines that k depends on temperature changes according to Eq. 10.
In which K0 is the pre-exponential term; Ea is the Activation energy (in kJ mol−1); R is the universal constant of ideal gases (8.314 J K−1 mol−1); T is the temperature in which the experiments were conducted (in K).
Based on the previous Eqs. 10 and 9, it was empirically defined that qCA0 is linearly dependent on temperature, so the Eq. 11 can be written as follows:
where manipulated variables: T = temperature (in K); t = time (in min); kinetic parameters are: K0 = kinetic constant (in min−1); Ea = activation energy [in J mol−1]; overall constants: R = universal constant of ideal gases (8.3144 J K−1 mol−1).
Process simulation
The simulations of the methane gas production process were carried out in the Aspen Plus™ software, where the simulations were run according to the conditions of the experimental design presented in Table 1. For this, the non-random two-liquid (NRTL) model was used as a thermodynamic package for the equilibrium calculations and the batch reactor was adopted in the reaction block to represent the biodigester, where the chemical reaction developed in the chemical reaction block was inserted in this reactor. The chemical reaction created made use of the mechanism presented in Eq. 2 (Arrhenius-type kinetic model) [18, 19], using the kinetic parameters obtained by the experimental results for the reaction of CH4 formation from the substrate (malt bagasse). This reaction model was inserted in the reactor block where data from the feed currents and the reactor operating conditions were used. Each reaction in the reactor was carried out at steady state. Twelve simulations were performed to each temperature condition, where these simulations were performed at the instant of time every 1500 s. At the end of all 12 simulations, the results were integrated into a single graph showing the behavior as a function of time (sensitivity analysis). A flowsheet was set up with a storage unit for biodigestion products (accumulator) and a gas burning unit for energy generation (boiler). The stopping criterion for the simulations was the residence time of 5 h (18,000 s), but the simulations respected the planning in Table 1.
Factorial design 23
The factorial design adopted for this work is described in Table 1, which shows the variables chosen for the study (process time, temperature and amount of substrate). Experimental planning considered the central point, thus a factorial design 23. This planning was used to perform the simulations, taking into account the activation energy and the kinetic constant obtained from the kinetic model. The amounts of substrate and water were calculated following the methodology adapted from Hansen et al. [20] and Alves [21], in which they consider 0.005 kg of substrate for 5.0 × 10–5 m3 of inoculum (sludge). The response variable was the yield (or conversion) of CH4 produced. Moreover, the data acquired in the factorial design 23 were evaluated at a 95% significance level in the Statistica ME™ Software. The minimum squares method was adopted to obtain the curve fit and the statistical parameters for model and data adjustment [22].
Surface response analysis was carried out by the graphs generated from Statistica software, which plotted the variation of CH4 conversion with the controlled variables (time, temperature and amount of substrate) [22]. Additionally, Pareto chart was also generated and considered the effect of each variable, as well as the combination of different ones, in the CH4 conversion considering a significance level of 95%. The pareto chart is a summary of the variables with most representative effects over the response variables (CH4 conversion), displayed in order of significance [22].
Results and discussion
Characterization of malt waste and industrial sludge
The number of volatile solids is directly related to the level of organic load, directly responsible for the production of biogas [9, 10]. The higher this value, the better the biodigestion/AD process. Another important factor in the production of biogas, which must be taken into account, is the carbon/nitrogen (C/N) ratio. The author also emphasizes that it is important that this ratio is not too low and that the proportion of nitrogen is lower than that of carbon, as the process can be easily affected due to the inhibition of bacteria caused by the release of ammonia, causing nitrogen deficiency in the process. The values observed in Table 2 show that both the substrate and the inoculum have characteristics that benefit the production of biogas. It is observed in Table 2 that the electrical conductivity increased from the initial to the final condition. Konrad et al. [23] state that the increase in electrical conductivity demonstrates that organic matter was being degraded anaerobically by bacteria.
The alkalinity results obtained from the samples before and after biodigestion show that the final substrate has a higher alkalinity value than the initial one, indicating that the anaerobic biodigestion process is responsible for the production of alkalizing substances, mainly bicarbonates that are responsible for neutralizing the acids produced, increase resistance to pH drop [10, 24] and maintain appropriate levels for better system performance.
According to Moraes et al. [24], volatile acids can be used as substrates by bacteria, but they can act as inhibitors, since at low pH values they preferentially form acids in non-ionized forms, which can penetrate the cell membrane and inhibit bacteria. Detailed investigation of the parameters of substrate characterization is presented in the section S1, in Supplementary Material. The results in Table S1 (supplementary material) show a reduction in volatile acidity, which corroborates the study by Moraes et al. [24]. Additionally, the increase in pH (or in electrical conductivity) demonstrates that the organic matter was being degraded anaerobically by the bacteria.
Kinetic behavior of biogas production
It can be seen in Fig. 3 that the behavior of biogas production is similar at different temperatures. However, methane production increased 20% when temperature rose from 293 to 298 K and increased 10% when temperature increased to 303 K. This is corroborated when a parallel is made with the optimal temperature range for the bacteria activity. Higher temperatures stimulate the growth of mesophilic bacteria responsible for methane formation, resulting in high efficiency processes and shorter retention times. Castillo et al. [25], evaluating the optimum operational conditions of the AD of municipal solid waste, found that the best temperature for stabilization of the process was 303 K, the temperature that presented the best performance in the present work.
At the beginning of AD, the sensor detected the production of CO2, this is the result of the acetogenesis phase in which there is the transformation of fatty acids into organic acid, releasing hydrogen and CO2, a process that precedes methanogenesis (methane production).
Figure 4 shows the results of biogas production carried out in the bench reactor for the temperature conditions studied along with the curves fitted to the proposed kinetic model. The global fit obtained a determination coefficient (R2) of 0.89 and values for the kinetic constant of 5.41 × 10–7 s−1 and Activation energy of 8.25 kJ mol−1. It can be seen from Fig. 3 that the temperature has an influence on the formation of methane gas in the process. The temperature curve of 293 K showed the lowest rate of methane gas formation while the temperature curve of 303 K was the one that showed the highest rate of methane gas formation. In the temperature range between 293 and 298 K, the rate of use of the substrate can be reduced, because in this range the activity of psychrotrophic bacteria is stronger. Under psychrophilic conditions, bacteria involved in AD process have their growth compromised and substrate utilization rate and biogas yield can be reduced [26]. The velocity of CH4 synthesis may also be related to the concentration of active microorganisms in the AD process.
Trommer et al. [27] investigated the kinetic behavior of CH4 decomposition to produce H2 and black matter in a vortex flow reactor. The authors found values for the Arrhenius kinetic parameters worked in the temperature range of 599–1499 K. Although these conditions are considerably greater from those used in this work, the order of magnitude of these parameters can be compared for extreme temperature conditions. Vadim D. Knyazev and collaborators carried out studies of the reactions of C2H3 + H2 ⇄ H + C2H4 and CH3 + H2 ⇄ H + CH4 using mass spectrometry analysis [28]. The rate constants were determined in time-resolved experiments as a function of temperature. The authors determined only the kinetic constant without considering the activation energy. Pichas et al. [29] studied the dry reforming of methane by carbon dioxide. The authors worked with the temperature range from 632 to 712 K. Although the study conditions are different from those studied in this work, one might have an idea of the order of magnitude of the activation energy for studies with CH4 under different process conditions. Table 3 describes a comparative assessment of AD process with different types of substrates.
Factorial design
Given the results, it was possible to plot the surface curves as shown below. Figure 5 presents the surface responses for the independent variables temperature (K) and time (s), and for the dependent variable “conversion” (in %). Experimental data of factorial design 23 for the AD are shown in Table S2 (supplementary material). From Fig. 5a is possible to notice that the higher the temperature, the greater the conversion. However, in comparison with the time of AD process, it presents itself in a more expressive way. The longer the process time, the greater the conversion of the substrate into CH4. It is possible to notice that the higher the biomass ratio (Fig. 5b), the lower is biogas conversion. In comparison with the variable “time”, this one presents a more influential factor in AD, where it is possible to notice that the longer is the operating time, the greater the conversion of substrate into gas in the formation of CH4. From Fig. 5c it is noted that the variables “biomass ratio” and “temperature” showed inverse influences on the formation of CH4. While the conversion decreases with the increase of the biomass ratio in aqueous medium, the variable temperature favors the substrate conversion.
From Pareto chart (Fig. 5d) the variable that most influenced the CH4 conversion was the time, followed by the variable relative to substrate composition (termed in this paper as biomass ratio) and temperature. The longer the substrate is retained in the digester, the greater amount of CH4 can be formed, as the activity of microorganisms can be maintained. The degradation efficiency is indicated by the percentage of organic material degraded and converted into biogas during a given time. All these variables being analyzed through linear models. It is possible to observe that for quadratic models only the time variable is representative although reaching a significance limit of 95%.
Computational simulation in Aspen Plus™
After analyzing the results, a simulation was performed with the best operating conditions for the experimental design presented in Table 4. A temperature of 303 K was used with a substrate ratio in the aqueous phase of 22.5 kg m−3 and an AD time of 7500 s as shown in Fig. 6, these data were taken from the library that feeds the Aspen Plus™ software. It was possible to obtain the profile of the vapor fraction as a function of pressure for the gas mixture produced by the reactor as shown in the graph of Fig. 6. It can be noted that the vapor fraction in the gas phase decreases as the pressure of the medium increases, obtaining 35% of methane in gaseous form at pressure 5.00 × 105 Pa. This is characteristic of the behavior of gases with high volatility such as methane, where the vapor pressure is 1.01 × 105 Pa at a temperature of − 161.5 °C (111.65 K), as informed in the Chemical Information Sheet in Supplementary Material, S4.
According to Aspen Plus™ simulations, for operating conditions of 303 K of temperature and with a ratio of substrate in aqueous phase of 22.5 kg m−3 and a process time of 7500 s (as described in the section S2 in Supplementary Material), an amount of energy generated of 0.0446 kW can be obtained. Making the staggering to serve a generator set with a capacity of 10.0 kWh, (40 kW in a batch of 14,400 s) an amount of biomass of 1.11 × 10–2 kg s−1 of malt bagasse in aqueous medium is required. This volume of malt bagasse is within the reality of small breweries that could use the gas produced to generate energy to supply the process or factory. From Fig. 6b, it is seen that the vapor fraction of the gas increases as the temperature increases, as an indicative of the increase in CH4 production, favored by higher temperatures.
According to factorial design experiments and the AD process simulations, temperature range simulated in this work (Table 5) encompasses the temperature range of several works found in the literature, which varied from 308 to 328 K [10, 30, 31]. This implies that both the simulation and the proposed kinetic model represent the behavior of biogas production considering the experimental condition established for 303 K (mesophilic methane fermentation). However, further investigations shall be carried out at greater temperature ranges (up to 328 K) to establish the kinetic parameters for thermophilic methane fermentation [32]. It was also observed that the process time is of fundamental importance, with a slow formation rate suggesting a long time for biogas production.
Conclusions
Through the results obtained in the work, it was possible to verify that the malt bagasse has a good potential for biogas generation. The characterization of the inoculum and the substrate showed good biodegradability and a good efficiency in the conversion of organic matter into biogas. The behavior of the gas generation curves over time showed a good methane production since the first days of retention. Through the performed analyses, it was observed that the temperature is an interference parameter in the process. In the conditions delimitated for the AD experiments, the higher the temperature, the higher the rate of methane formation and the more volume of biogas was generated, corroborating the literature. The influence of temperature could be verified in the bench experiment, in the kinetic modeling, in the simulation and in the surface and Pareto graphs. Nevertheless, novel AD experiments might be performed at greater temperatures to establish the kinetics parameters in the thermophilic fermentation with more precision. The parameters of the kinetic model were acquired following the Arrhenius equation and were 5.42 × 10–7 s−1 for the kinetic constant, and 8.25 kJ mol−1 for the activation energy.
The sensitivity analysis performed by the simulation indicated that the temperature had an important factor for the formation of gas at the end of the process. As for the ratio of substrate in aqueous medium, an inverse situation was observed as for the temperature, the higher the substrate/water ratio, the lower the conversion into gas, showing that the amount of water used in the substrate interferes with the production of biogas.
Statistical study through response surface graphics indicated that the optimal temperature range for the process operation was between 303 and 323 K, showing that above this value, the process is tending to steady state. The analysis performed by the Pareto chart showed that the process time is of fundamental importance, indicating that the longer the substrate is retained in the biodigester, the greater the amount of methane gas formed. This is because the activity of the microorganism can be kept at work. The substrate/water ratio is the second most important variable followed by temperature.
Finally, it was found that an amount of biomass of 1.11 × 10–2 kg s−1 of malt bagasse in aqueous medium can produce enough methane gas to supply an electric power generator with a capacity of 10.0 kWh. This volume of malt bagasse is within the reality of small breweries where they could use the gas produced to generate energy. This energy consumed in the industry by the generation of biogas from its own residues would help to reduce the environmental impact in an economically favorable way, fulfilling ESG practices.
Data availability
Any complementary material, as well as the research data, might be provided by contacting any of the authors under reasonable request.
References
Amienyo D, Azapagic A (2016) Life cycle environmental impacts and costs of beer production and consumption in the UK. Int J Life Cycle Assess 21:492–509. https://doi.org/10.1007/s11367-016-1028-6
Villacreces S, Blanco CA, Caballero I (2022) Developments and characteristics of craft beer production processes. Food Biosci 45:101495. https://doi.org/10.1016/j.fbio.2021.101495
Lindenberger H (2021) What does 2021 look like for craft beer? Forbes. https://www.forbes.com/sites/hudsonlindenberger/2021/01/20/what-does-2021-look-like-for-craft-beer/?sh=4130be275daf. Accessed 23 Apr 2022
Franciski MA, Peres EC, Godinho M, Perondi D, Foletto EL, Collazzo GC, Dotto GL (2018) Development of CO2 activated biochar from solid wastes of a beer industry and its application for methylene blue adsorption. Waste Manag 78:630–638. https://doi.org/10.1016/j.wasman.2018.06.040
de Oliveira LRG, dos Santos Filho DA, Fraga TJM, Jucá JFT, da Motta Sobrinho MA (2021) Kinetics assessment and modeling of biogas production by anaerobic digestion of food wastes and acclimated sewage sludge. J Mater Cycles Waste Manag 23:1646–1656. https://doi.org/10.1007/s10163-021-01248-x
Jeppu GP, Janardhan J, Kaup S, Janardhanan A, Mohammed S, Acharya S (2022) Effect of feed slurry dilution and total solids on specific biogas production by anaerobic digestion in batch and semi-batch reactors. J Mater Cycles Waste Manag 24:97–110. https://doi.org/10.1007/s10163-021-01298-1
Feiz R, Johansson M, Lindkvist E, Moestedt J, Påledal SN, Ometto F (2022) The biogas yield, climate impact, energy balance, nutrient recovery, and resource cost of biogas production from household food waste—a comparison of multiple cases from Sweden. J Clean Prod 378:134536. https://doi.org/10.1016/j.jclepro.2022.134536
Agarwal NK, Kumar M, Ghosh P, Kumar SS, Singh L, Vijay VK, Kumar V (2022) Anaerobic digestion of sugarcane bagasse for biogas production and digestate valorization. Chemosphere 295:133893. https://doi.org/10.1016/j.chemosphere.2022.133893
Chae KJ, Jang AM, Yim SK, Kin IS (2008) The effects of digestion temperature and temperature shock on the biogas yields from the mesophilic anaerobic digestion of swine manure. Bioresour Technol 99:1–6. https://doi.org/10.1016/j.biortech.2006.11.063
dos Santos Filho DA, Oliveira LRG, Fraga TJM, da Motta Sobrinho MA, Juca JFT (2020) Development of a horizontal reactor with radial agitation to synthesize bio-methane from biomass waste and domestic sewage sludge. J Clean Prod 257:120616. https://doi.org/10.1016/j.jclepro.2020.120616
International Energy Agency (2017) World Energy Balances: Overview. [S.l.]
Arvidsson S, Dumay J (2022) Corporate ESG reporting quantity, quality and performance: where to now for environmental policy and practice? Bus Strat Environ 31:1091–1110. https://doi.org/10.1002/bse.2937
Hadj B (2021) Nonlinear impact of biomass energy consumption on ecological footprint in a fossil fuel–dependent economy. Environ Sci Pollut Res 28:69329–69342. https://doi.org/10.1007/s11356-021-15521-x
Dyer AC, Nahil MA, Williams PT (2022) Biomass: polystyrene co-pyrolysis coupled with metal-modified zeolite catalysis for liquid fuel and chemical production. J Mater Cycles Waste Manag 24:477–490. https://doi.org/10.1007/s10163-021-01334-0
Tanzil AH, Brandt K, Zhang X, Wolcott M, Silva Lora EE, Stockle C, Garcia-Perez M (2022) Evaluation of bio-refinery alternatives to produce sustainable aviation fuels in a sugarcane mill. Fuel 321:123992. https://doi.org/10.1016/j.fuel.2022.123992
Khan IU, Othman MHD, Hashim H, Matsuura T, Ismail AF, Rezaei-DashtArzhandi M, Wan Azelee I (2017) Biogas as a renewable energy fuel—a review of biogas upgrading, utilisation and storage. Energy Convers Manag 150:277–294. https://doi.org/10.1016/j.enconman.2017.08.035
Passos F, Cordeiro PHM, Baeta BEL, de Aquino SF, Perez-Elvira SI (2018) Anaerobic co-digestion of coffee husks and microalgal biomass after thermal hydrolysis. Bioresour Technol 253:49–54. https://doi.org/10.1016/j.biortech.2017.12.071
Fogler HS (2020) Elements of chemical reaction engineering, 6th edn. Pearson, London
Levenspiel O (1998) Chemical reaction engineering, 3rd edn. Wiley, New Jersey
Hansen TL, Schmidt JE, Angelidaki I, Marca E, Jansen JC, Mosbæk H, Christensen TH (2004) Method for determination of methane potentials of solid organic waste. Waste Manag 24:393–400. https://doi.org/10.1016/j.wasman.2003.09.009
Alves IRFS (2008) Análise experimental do potencial de geração de biogás em resíduos sólidos urbanos, Experimental analysis of the potential for generating biogas in urban solid waste (in Portuguese). Dissertation, Federal University of Pernambuco
Barros Neto B, Scarminio IS, Bruns RE (2010) Como fazer experimentos. Pesquisa e Desenvolvimento na Ciência e na Indústria, How to perform experiments. Research and development in Science and industry (in Portuguese), 4th Ed. Campinas, Bookman
Konrad O, Bezama AB, Prade T, Backes GM, Oechsner H (2016) Enhancing the analytical capacity for biogas development in Brazil: assessment of an original measurement system for low biogas flow rates out of agricultural biomass residues. J Braz Assoc Agric Eng 36(5):792–798. https://doi.org/10.1590/1809-4430-Eng.Agric.v36n5p792-798/2016
Moraes BS, Zaiat M, Bonomi A (2015) Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: challenges and perspectives. Renew Sust Energy Rev 44:888–903. https://doi.org/10.1016/j.rser.2015.01.023
Castillo MEF, Cristancho DE, Victor Arellano A (2006) Study of the operational conditions for anaerobic digestion of urban solid wastes. Waste Manag 26(5):546–556. https://doi.org/10.1016/j.wasman.2005.06.003
Trzcinski AP, Stuckey DC (2010) Treatment of municipal solid waste leachate using a submerged anaerobic membrane bioreactor at mesophilic and psychrophilic temperatures: analysis of recalcitrants in the permeate using GC-MS. Water Res 44(3):671–680. https://doi.org/10.1016/j.watres.2009.09.043
Trommer D, Hirschb A, Steinfel D (2004) Kinetic investigation of the thermal decomposition of CH4 by direct irradiation of a vortex-flow laden with carbon particles. Int J Hydrog Energy 29:627–633. https://doi.org/10.1016/j.ijhydene.2003.07.001
Knyazev VD, Bencsura A, Stoliarov SI, Slagle IR (1996) Kinetics of the C2H3 + H2 ⇄ H + C2H4 and CH3 + H2 ⇄ H + CH4 reactions. J Phys Chem 100(27):11346–11354. https://doi.org/10.1021/jp9606568
Pichas C, Pomonis P, Petrakis D, Ladavos A (2010) Kinetic study of the catalytic dry reforming of CH4 with CO2 over La2−x SrxNiO4 perovskite-type oxides. Appl Cat A 386:116–123. https://doi.org/10.1016/j.apcata.2010.07.043
Zhang Q, Hu J, Lee D (2016) Biogas from anaerobic digestion processes: research updates. Renew Energy 98:108–119. https://doi.org/10.1016/j.renene.2016.02.029
Fezzani B, Ben Cheikh R (2010) Two-phase anaerobic co-digestion of olive mill wastes in semi-continuous digesters at mesophilic temperature. Bioresour Technol 101:1628–1634. https://doi.org/10.1016/j.biortech.2009.09.067
Kim JK, Oh BR, Chun YN, Kim SW (2006) Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J Biosci Bioeng 102:328–332. https://doi.org/10.1263/jbb.102.328
Funding
This work was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [Grant number 304934/2021-6], the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) [Grant number 88887.616594/2021-00] and the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) [Grant Numbers APQ-0167-3.07/21 and DCR-0040-3.07/21].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [ICPT]. The first draft of the manuscript was written by [ICPT] and [TJMF] and all authors commented on previous versions of the manuscript. The visualization and revisions of graphics were carried out by [TJMF], [ICPT] and [MPdS]. The supervision of experimental protocols and results was carried out by [GCBC] and [TPCS]. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
The authors declare that they did not perform research involving human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tschoeke, I.C.P., Fraga, T.J.M., da Silva, M.P. et al. Biogas production from malt bagasse from craft beer industry: kinetic modeling and process simulation. J Mater Cycles Waste Manag 25, 2809–2821 (2023). https://doi.org/10.1007/s10163-023-01715-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01715-7